Abstract
The effectiveness of combining mitomycin C (MMC), tamoxifen (TAM), and 1-(2-tetrahydrofuryl)-5-fluorouracil (tegafur) was evident in patients with estrogen receptor-positive (ER+) breast cancers. UFT, an oral preparation of tegafur and uracil at a molar ratio of 1:4, was reported to have higher antitumor effects than tegafur alone for patients with breast cancer. Therefore, the combined chemotherapy of MMC, TAM and UFT may possibly be effective for breast cancer.
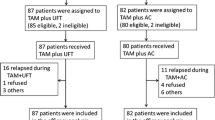
From 1988 to 1991, we studied the effects of postoperative adjuvant therapy for Japanese women with stage II breast cancer, all seen at 71 institutions in western areas of Japan. Five hundred and ninety four patients with stage II primary breast cancer who had undergone curative surgery, including total mastectomy and axillary lymph node dissection, were enrolled. On the day of surgery, each patient was given 13 mg/m2 of MMC intravenously. Patients with ER+ tumors were then assigned to group A or group B. Group A received 30 mg/day of TAM given orally from postoperative 2 weeks, for 2 years. Group B was additionally given an oral dose of 300 mg/day of UFT for 2 years, given concomitantly with 30 mg/day of TAM. Patients with ER− tumors were assigned to group C or group D. Group C were prescribed 300 mg/day of UFT, orally, from postoperative 2 weeks for 2 years, and group D were additionally given an oral dose of 30 mg/day of TAM together with 300 mg/day of UFT.
There were no differences among the groups regarding prognostic factors or doses of MMC and TAM in ER+ patients and MMC and UFT in ER− patients. Toxicity rates for leukopenia, anorexia, and nausea/vomiting were higher in group B than in group A patients. There were no statistical differences in the overall survival and disease–free survival times between groups A and B, or groups C and D, for all eligible cases. In a retrospective subgroup analysis using Bonferroni's adjustments, the additional effect of UFT on the combined treatment of MMC and TAM lengthened the disease-free survival time for patients with premenopausal ER+ cancers (corrected P value by Bonferroni's adjustments <0.05). Multivariate analysis showed that effects of the combined treatment of MMC, TAM, and UFT was significantly related to the menopausal status (P<0.0 1).
Our findings show that postoperative ingestion of MMC, TAM, and UFT was effective for patients with premenopausal ER+ stage II breast cancer.
Similar content being viewed by others
References
Consensus conference: Adjuvant chemotherapy for breast cancer. JAMA 254: 3461–3463, 1985
Pritchard KI: Current status of adjuvant endocrine therapy for resectable breast cancer. Semin Oncol 14: 23–33, 1987
Goldhirsch AG, Wood WC, Senn H-J, Glick JH, Gelber RD: Meeting highlights: International consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst 87: 1441–1445, 1995
Patterson I, Furr B, Wakeling A, Battersby L: The biology and physiology of 'Nolvadex' _(tamoxifen) in the treatment of breast cancer. Breast Cancer Res Treat 2: 363–374, 1982
Early Breast Cancer Trialists' Collaborative Group: Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: An overview of 61 randomized trials among 28,896 women. New Engl J Med 319: 1681–1691, 1988
Fisher B, Redmond C, Legault-Poisson S, Dimitrov NV, Brown AM, Wickerham DL, Wolmark N, Margolese RG, Bowman D, Glass AG, Kardinal CG, Robidoux A, Jochimsen P, Cronin W, Deutsch M, Fisher ER, Myers DB, Hoehn JL: Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: Results from the National Surgical Adjuvant Breast and Bowel Projects B-16. J Clin Oncol 8: 1005–1018, 1990
Early Breast Cancer Trialist's Collaborative Group: Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomized trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 339: 1–15, 71-84, 1992
Bonadonna G, Valagussa P, Moliterini A, Zambetti M, Brambilla C: Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. New Engl J Med 332: 901–906, 1995
Yoshida M, Murai H, Miura H: Chemotherapy with mitomycin-C and cyclophosphamide adjuvant to surgery for breast cancer. Jpn J Clin Oncol 9: 27–34, 1979
Koyama H, Wada T, Takahashi Y, Nishizawa Y, Iwanaga T, Aoki Y, Terasawa T, Kosaki G, Kajita A, Wada A: Surgical adjuvant chemotherapy with mitomycin C and cyclophosphamide in Japanese patients with breast cancer. Cancer 46: 2373–2379, 1980
Ikenaka K, Shirasaka T, Kitano S, Fujii S: Effect of uracil on metabolism of 5-fluorouracil in vitro. Gann 70: 353–359, 1979
Maehara Y, Nagayama S, Okazaki H, Nakamura H, Shirasaka T, Fujii S: Metabolism of 5-fluorouracil in various human normal and tumor tissues. Gann 72: 824–827, 1981
Etienne MC, Chéradame S, Fischel JL, Formento FP, Dassonville O, Renée N, Schneider M, Thyss A, Demard F, Milano G: Response to fluorouracil therapy in cancer patients: The role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol 13: 1663–1670, 1995
Heggie GD, Sommadossi J-P, Cross DS, Huster WJ, Diasio RB: Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 47: 2203–2206, 1987
Allegra JC, Lippman ME, Thompson EB, Simon R: An association between steroid hormone receptors and response to cytotoxic chemotherapy in patients with metastatic breast cancer. Cancer Res 38: 4299–4304, 1978
Lippman ME, Allegra JC, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HMT, Aitken SC, Warren R: The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. New Engl J Med 298: 1223–1228, 1978
Maehara Y, Emi Y, Sakaguchi Y, Kusumoto T, Kakeji Y, Kohnoe S, Sugimachi K: Estrogen-receptor-negative breast cancer tissue is chemosensitive in vitro compared with estrogen-receptor-positive tissue. Eur Surg Res 22: 50–56, 1990
Wada T, Koyama H, Nishizawa Y, Takahashi Y, Fukuda I, Iwanaga T, Terasawa T: Chemo-endocrine therapy for advanced breast cancer-A combined treatment with tamoxifen and FT-207. J Jpn Soc Cancer Ther 16: 51–55, 1981 (in Japanese with English abstract)
Morimoto T, Ogawa M, Orita K, Sugimachi K, Toge T, Dohi K, Nomura Y, Monden Y, Ogawa N: Postoperative adjuvant randomized trial comparing chemoendocrine therapy, chemotherapy and immunotherapy for patients with stage II breast cancer: 5-year results from the Nishinihon cooperative study group of adjuvant chemoendocrine therapy for breast cancer (ACETBC) of Japan. Eur J Cancer 32A: 235–242, 1996
Fujii S, Ikenaka K, Fukushima M, Shirasaka T: Effect of uracil and its derivatives on antitumor activity of 5-fluorouracil and 1-(2-tetrahydrofuryl)-5-fluorouracil. Gann 69: 763–772, 1978
Fujii S, Kitano S, Ikenaka K, Shirasaka T: Effect of coadministration of uracil or cytosine on the antitumor activity of clinical doses of 1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in rodents. Gann 70: 209–214, 1979
Ota K, Taguchi T, Kimura K. Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol 22: 333–338, 1988
Tashiro H, Nomura Y and Ohsaki A: A double blind comparative study of tegafur (FT) and UFT (a combination of tegafur and uracil) in advanced breast cancer. Jpn J Oncol 24: 212–217, 1994
Baum M, Brinkley DM, Dossett JA, McPherson K, Patterson JS, Rubens RD, Smiddy FG, Stoll BA, Wilson A, Lea JC, Richards D, Ellis SH: Controlled trial of tamoxifen as adjuvant agent in management of early breast cancer. Lancet 1(8319): 257–261, 1983
Japanese Breast Cancer Society: The general rules for clinical and pathological recording of breast cancer. Jpn J Surg 19: 612–632, 1989
Japan Society for Cancer Therapy: Criteria for the evaluation of the clinical effects of solid cancer chemotherapy. J Jpn Soc Cancer Ther 28: 101–130, 1993
Dixon WJ (ed): BMDP Statistical Software. University of California Press Berkeley, CA, 1988
Rosner B: Fundamentals of Biostatistics. Duxbury Press, New York, 1995
Cox DR: Regression models and life tables. J Royal Statist Soc Series B 34: 187–220, 1972
Izuo M: Clinicopathological features of recurrent breast cancer. Jpn J Cancer Chemother 12: 412–420, 1985 (in Japanese with English abstract).
Rossi A, Bonadonna G, Valagussa P, Veronesi U: Multimodal treatment in operable breast cancer: five-year results of the CMF programme. Br Med J 282: 1427–1431, 1981
Fisher B, Fisher ER, Redmond C: Ten-year results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trial evaluating the use of L-phenylalanine mustard (L-PAM) in the management of primary breast cancer. J Clin Oncol 4: 929–941, 1986
Budzar AU, Kau SW, Smith TL, Hortobagyi GN: Ten-year results of FAC adjuvant chemotherapy trial in breast cancer. Am J Clin Oncol 12: 123–128, 1989
Richards MA, O'Reilly SM, Howell A, George WD, Fentiman IS, Chaudary MA, Crowther D, Rubens RD: Adjuvant cyclophosphamide, methotrexate, and fluorouracil in patients with axillary node-positive breast cancer: An update of the Guy's/ Manchester trial. J Clin Oncol 8: 2032–2039, 1990
Early Breast Cancer Trialists' Collaborative Group: Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. Lancet 339: 71–85, 1992
Osborne CK, Hobbs K, Clark GM: Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res 45: 584–590, 1985
Conte PF, Frashini G, Alama A, Nicolin A, Corsaro E, Canavese G, Rosso R, Drewinko B: Chemotherapy following estrogen induced expansion of the growth fraction of human breast cancer. Cancer Res 45: 5926–5930, 1985
Hug V, Johnston D, Finders M, Hortobagyi G: Use of growth stimulatory hormones to improve the in vitro therapeutic index of doxorubicin for human breast tumors. Cancer Res 46: 147–152, 1986
Elston CW, Gresham GA, Rao GS, Zebro T, Haybittle JL, Houghton J, Kearney G: The cancer research campaign (King's/ Cambridge) trial for early breast cancer: Clinicopathological aspects. Br J Cancer 45: 655–669, 1982
Early breast cancer trialist's collaborative group: Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351: 1451–1467, 1998
Tormey DC, Gray R, Gilchrist K, Grage T, Carbone PP, Wolter J, Woll JE, Cummings FJ: Adjuvant chemohormonal therapy with cyclophosphamide, methotrexate, 5-fluorouracil, and prednisone (CMFP) or CMFP plus tamoxifen compared with CMF for premenopausal breast cancer patients. Cancer 65: 200–206, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sugimachi, K., Maehara, Y., Akazawa, K. et al. Postoperative chemo–endocrine treatment with mitomycin C, tamoxifen, and UFT is effective for patients with premenopausal estrogen receptor–positive stage II breast cancer. Breast Cancer Res Treat 56, 111–122 (1999). https://doi.org/10.1023/A:1006221425652
Issue Date:
DOI: https://doi.org/10.1023/A:1006221425652