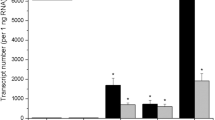
Abstract
We have isolated and characterized the genomic clone of maize casein kinase 2 (CK2) α subunit using the previously described αCK2-1 cDNA clone as a probe. The genomic clone is 7.5 kb long and contains 10 exons, separated by 9 introns of different size, two larger than 1.5 kb and the others around 100–150 bp. The sequence of the exons is 100% homologous to the sequence of the αCK2-1 cDNA. Southern hybridization of total genomic DNA from maize embryos with αCK2 cDNA indicated that the αCK2-1 gene is part of a multigenic family. We also isolated a new embryo cDNA clone coding for an αCK2-2 subunit. We studied the regulation of the enzyme in embryos at the mRNA level, at the protein level and by activity testing. By using immunocytochemistry the CK2 protein was localized in several types of cells of mature embryos. Particularly strong signals were visible in the cytoplasm of epidermis and meristematic cells. Decoration of nuclei of root cortex and scutellum cells was also observed suggesting that CK2 can shift from the cytoplasm into nuclei in specific cell types. We examined whether CK2 contained specific protein domains which actively target the protein to the nucleus by using in-frame fusions of the maize CK2 α subunit to the reporter gene encoding β-glucuronidase (GUS) which were assayed in transiently transformed onion epidermal cells. Analysis of chimeric constructs identified one region containing a nuclear localization signal (NLS) that is highly conserved in other αCK2 proteins.
Similar content being viewed by others
References
Ackerman, P., Claiborne, V.C.G. and Osheroff, N. 1985. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc. Natl. Acad. Sci. USA 82: 3164–3168.
Belenguer, P., Baldin, V., Mathieu, C., Prats, H., Bensald, M., Bouche, G. and Amalric, F. 1989. Protein kinase NII and the regulation of rDNA transcription in mammalian cells. Nucl. Acids Res. 17: 6625–6636.
Boldyreff, B., Meggio, F., Dobrowolska, G., Pinna, L.A. and Issinger O.-G. 1993. Expression and characterization of a recombinant maize CK-2 α subunit. Biochim. Biophys. Acta 1173: 32–38.
Boyle, W.J., Smeal, T., Defize, L.H.K., Angel, P., Woodgett, J.R., Karin, M. and Hunter, T. 1991. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 64: 573–584.
Brown, J.W.S. 1986. A catalogue of splice junctions and putative branch point sequences from plant introns. Nucl. Acids Res. 14: 9549–9559.
Chen-Wu, J.L.P., Padmanabha, R. and Glover, C.V.C. 1988. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol. Cell. Biol. 8: 4981–4990.
Cochet, C. and Chambaz, E.M. 1983. Oligomeric structure and catalytic activity of G type casein kinase, isolation of the two subunits and renaturation experiments. J. Biol. Chem. 258: 1403–1406.
Collinge, M.A. and Walker, J.C. 1994. Isolation of an Arabidopsis thaliana casein kinase II β subunit by complementation in Saccharomyces cerevisiae. Plant Mol. Biol. 25: 649–658.
Dobrowolska, G., Meggio, F., Szczegielniak, J., Muszynska, G. and Pinna, L.A. 1992. Purification and characterization of maize seedling casein kinase IIB, a monomeric enzyme immunologically related to the α subunit of animal casein kinase-2. Eur. J. Biochem. 204: 299–303.
Dobrowolska, G., Boldyreff, B. and Issinger, O.-G.: Cloning and sequencing of the casein, kinase 2 α subunit from Zea mays. Biochim. Biophys. Acta 1129: 139–140.
Edelman, A.M., Blumenthal, D.A. and Krebs, E.G. 1987. Protein serine and threonine kinases. Annu. Rev. Biochem. 56: 563–613.
Espunya, M.C. and Martinez, M.C. 1997. Identification of two different molecular forms of A. thaliana casein kinase II. Plant Sci. 124: 131–142.
Fobert, P.R., Gaudin, V., Lunness, P., Coen, E.S. and Doonan, J.H. 1996. Distinct classes of the cdc2-related genes are differentially expressed during the cell division cycle in plants. Plant Cell 8: 1465–1476.
Garcia-Bustos, J., Heitman, J. and Hall, M.N. 1991. Nuclear protein localization. Biochim. Biophys. Acta 1071: 83–101.
Goday, A., Jensen, A.B., Culiañez-Macià, F.B., Albà, M.M., Figueras, M., Serratosa, J., Torrent, M. and Pagès, M. 1994. The maize abscisic acid-responsive protein rab 17 is located in the nucleus and interacts with nuclear localization signals. Plant Cell 6: 351–360.
Goday, A., Sánchez-Martínez, D., Gómez, J., Puigdoménech, P. and Pagès, M. 1988. Gene expression in developing Zea mays embryos. Regulation by abscisic acid of a highly phosphorylated 23–25 kD group of proteins. Plant Physiol. 88: 564–569.
Goff, S.A., Klein, T.M., Roth, B.A., Fromm, M.E., Cone, K.C., Radicalla, J.P. and Chandler, V.L. 1990. Transactivation of the anthocyanin biosynthetic genes following transfer of B regulatory genes into maize tissues. EMBO J. 9: 2517–2522.
Hathaway, G.M. and Traugh, J.A. 1982. Casein kinasesmultipotential protein kinases. Curr. Top. Cell. Regul. 21: 101–127.
Hériché, J.K., Lebrin, F., Rabilloud, T., Leroy, D., Chambaz, E.M. and Goldberg, Y. 1997. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276: 952–955.
Hu, E. and Rubin C.S. 1991. Casein kinase II from Caenorhabditis elegans. Cloning characterization and developmental regulation of the gene encoding the β subunit. J. Biol. Chem. 266: 19796–19802.
Issinger, O.G. 1993. Casein kinases: pleiotropic mediators of cellular regulation. Pharm. Ther. 59: 1–30.
Jedlicki, A., Hinrichs, M.V., Allende, C. and Allende, J.E. 1992. The cDNA coding for the α and β subunits of Xenopus laevis is casein kinase II. FEBS Lett. 297: 280–284.
Jensen, A.B., Goday, A., Figueras, M., Jessop A.C. and Pagès, M. 1998. Phosphorylation mediates the nuclear targeting of the maize Rab 17 protein. Plant J.
Jefferson, R.A., 1987. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 5: 387–405.
Kalderon D, Roberts BL, Richardson, W.D. and Smith, A.E. 1984. A short amino acid sequence able to specify nuclear location. Cell 39: 499–509.
Kikkawa, U., Mann, S.K.O., Firtel, R.A. and Hunter, T. 1992. Molecular cloning of casein kinase II α subunit from Dictyostelium discoideum and its expression in the life cycle. Mol. Cell. Biol. 12: 5711–5723.
Klimczak, L.J., Farini, D., Giuliano, G., Walker, J.C. and Cashmore, A.R. 1995. Reconstitution of Arabidopsis casein kinase II from recombinant subunits and phosphorylation of transcription factor GBF1. Plant Cell 7: 105–115.
Klimczak, L.J., Schindler, U. and Cashmore, A.R. 1992. DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. Plant Cell 4: 87–98.
Krek, W., Maridor, G. and Nigg, E.A. 1992. Casein kinase II is a predominantly nuclear enzyme. J. Cell. Biol. 116: 43–55.
Li, H. and Roux, S.J. 1992a. Casein kinase II protein kinase is bound to lamina-matrix and phosphorylates lamin-like protein in isolated pea nuclei. Proc. Natl. Acad. Sci. USA 89: 8424–8432.
Li, H. and Roux S.J. 1992b. Purification and characterization of a casein kinase 2-type protein kinase from pea nuclei. Plant Physiol. 99: 686–692.
Lozeman, F.J., Litchfield, D.W, Piening, C., Takio, K., Walsh, K.A. and Krebs, E.G 1990. Isolation and characterization of human cDNA clones encoding the α and ά0 subunits of casein kinase II. Biochemistry 29: 8436–8447.
Lüscher, B., Christenson, E., Litchfield, D.W., Krebs, E.G. and Eisenman, R.N. 1990. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature 344: 517–522.
Maridor, G., Park, W., Krek, W. and Nigg, E.A. 1991. Casein kinase II. cDNA sequences, developmental expression, and tissue distribution of mRNA for α, ά0 and β subunits of the chicken enzyme. J. Biol. Chem. 266: 2362–2368.
Matsumoto, S., Mizoguchi, T., Oizumi, N., Tsuruga, M., Shinozaki, K., Taira, H. and Ejiri, S. 1993. Analysis of phosphorylation of wheat elongation factor 1b and 1b' by casein kinase II. Biosci. Biotechnol. Biochem. 57: 1740–1742.
Mizoguchi, T., Yamaguchi-Shinozaki, K., Hayashida, N., Kamada, H. and Shinozaki, K. 1993. Cloning and characterization of two cDNAs encoding casein kinase II catalytic subunits in Arabidopsis thaliana. Plant Mol. Biol. 21: 279–289.
ole-MoiYoi, O.K., Sugimoto, C., Conrad, P.A. and Macklin, M.D. 1992. Cloning and characterization of the casein kinase II α subunit gene from the lymphocyte-transforming intracellular protozoan parasite Theileria parva. Biochemistry 31: 6193–6202.
Padmanabha, R., Chen-Wu, J.L.P., Hanna, D.E. and Glover C.V.C. 1990. Isolation, sequencing and disruption of the CKA2 gene: Casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol. Cell. Biol. 10: 4089–4099.
Perez, M., Grande, J. and Itarte, E. 1987. Developmental changes in rat hepatic casein kinase 1 and 2. Eur. J. Biochem. 170: 493–498.
Pinna, L.A. 1987. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim. Biophys. Acta 1054: 267–284.
Pla, M., Goday, A., Vilardell, J., Gomez J. and Pagès M. 1989. Differential regulation of ABA-induced 23–25 kDa proteins in embryo and vegetative tissues of the viviparous mutants of maize. Plant Mol. Biol. 13: 385–394.
Plana, M., Itarte, E., Eritja, R., Goday, A., Pagès, M. and Martinez, M.C. 1991. Phosphorylation of maize rab 17 protein by casein kinase 2. J. Mol. Chem. 266: 22510–22514.
Prigent, C., Lasko, D.D., Kodama, K., Woodgett, J. and Lindahl, T. 1992. Activation of mammalian DNA ligase I through phosphorylation by casein kinase II. EMBO J. 11: 2925–2933.
Prywes, R., Dutta, A., Cromlish, J.A. and Roeder, R.G. 1988. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-fos enhancer. Proc. Natl. Acad. Sci. USA 85: 7206–7210.
Pyerin, W. 1994. Human casein kinase II: structures, genes, expression and requirement in cell growth stimulation. Adv. Enzym. Regul. 34: 225–246.
Raikhel, N.V. 1993. Nuclear targeting in plants. Plant Physiol. 100: 1627–1632.
Roux, S.J. 1993. Casein kinase-2-type protein kinases in plants: possible targets of polyamine action during growth regulation? Plant Growth Regul. 12: 193–197.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger, F., Nicklen, S. and Coulson, A.R. 1977. DNA sequencing with chain-termination inhibitors. Proc. Natl. Acad. Sci. USA 74: 5463–5467.
Saxena, A., Padmanabha, R. and Glover, C.V.C. 1987. Isolation and sequencing of cDNA clones encoding the alpha and beta subunits of drosophila casein kinase II. Mol. Cell. Biol. 7: 3409–3417.
Shieh, M.W., Wessler, S.R. and Raikhel, N.V. 1993. Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol. 101: 353–361.
Shneider, H.R., Reichert, G.H. and Issinger, O.-G. 1986. Enhanced casein kinase II activity during mouse embryogenesis: identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos. Eur. J. Biochem. 161: 733–738.
Shore, L.J., Peralta Soler, A. and Gilmour, S.K. 1997. Ornithine decarboxylase expression leads to translocation and activation of protein kinase CK2 in vivo. J. Biol. Chem. 272: 12536–12543.
van der Krol, A.R. and Chua, N.H. 1991. The basic domain of plant B-ZIP proteins facilitates import of a reporter protein into plant nuclei. Plant Cell 3: 667–675.
Varagona, M.J. and Raikhel, N.V. 1994. The basic domain in the bZIP regulatory protein Opaque-2 serves two independent functions: DNA binding and nuclear localization. Plant J. 5: 207–214.
Varagona, M.J., Schmidt, R.J. and Raikhel, N.V. 1992. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein, Opaque-2. Plant Cell 4: 1213–1227.
Vilardell, J., Goday, A., Freire, M.A., Torrent, M., Martinez, C., Torne, J.M. and Pagès, M. 1990.. Gene sequence, developmental expression, and protein phosphorylation of rab-17 inmaize. Plant Mol. Biol. 14: 423–432.
Zhang, S., Jin, C.-D. and Roux, S.J. 1993. Casein kinase II-type protein kinase from pea cytoplasm and its inactivation by alkaline phosphatase in vitro. Plant Physiol. 103: 955–962.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peracchia, G., Jensen, A.B., Culiáñez-Macià, F.A. et al. Characterization, subcellular localization and nuclear targeting of casein kinase 2 from Zea mays. Plant Mol Biol 40, 199–211 (1999). https://doi.org/10.1023/A:1006196530079
Issue Date:
DOI: https://doi.org/10.1023/A:1006196530079