Abstract
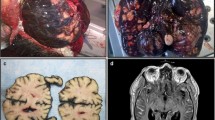
We experienced a rare case of leptomeningeal melanomatosis. The proliferative activity and nuclear accumulation of p53 in this tumor were examined, since the relationship between this tumor type and growth has not yet been elucidated. A 33-year-old Japanese man was shown to have leptomeningeal melanomatosis with multiple cutaneous pigmented nevi. The autopsy findings showed the presence not only of benign diffuse melanosis of the leptomeninges but also of leptomeningeal melanomatosis in the subarachnoid space and brain parenchyma. In the brain parenchyma, the direct invasion of tumor cells from the subarachnoid space and Virchow–Robin spaces filled with melanoma cells were observed. Multiple hemorrhagic areas invaded by melanoma cells were also present. Immunohistochemical staining with a monoclonal antibody to melanoma cells showed positivity in the tumor cells. Proliferation analysis using the MIB-1 antibody demonstrated that the labeling index of tumor cells invading brain parenchyma (2.54%) was higher than that in other lesions of the inner (0.89%) and outer layer (0.76%) of the subarachnoid space. Nuclear accumulation of p53 protein was rarely seen in the tumor cells.
We reported a case of leptomeningeal melanomatosis. Higher proliferative activity was found in invading cells of the brain parenchyma. Malignant transformation of the tumor did not appear to be associated with p53 gene mutation.
Similar content being viewed by others
References
Kadonaga JN, Frieden IJ: Neurocutaneous melanosis: Defi-nition and review of the literature. J Am Acad Dermatol 24: 747–755, 1991
Rokitansky J: —Ein ausgezeichneter Fall von Pigmentmal mit ausgebreiter Pigmentierung der inneren hirn-und Ruckenmarkshaut. Allg Wien Med Z 6: 113–116, 1861
Virchow R: Pigment und diffuse Melanose der Arachnoides. Virchows Archiv 16: 180–182, 1859
DeDavid M, Orlow SJ, Provost N, Marghoob AA, Roa BK, Wasti Q, Huang CL, Kopf AW, Bart RS: Neurocutaneous melanosis: Clinical features of large congenital melanocytic nevi in patients with manifest central nervous system melanosis. J Am Acad Dermatol 35: 529–538, 1996
Savitz MH, Anderson PJ: Primary melanoma of the leptomeninges: A review. Mount Sinai J Med 41: 774–791, 1974
Gown AM, Vogel AM, Hoak D, Gouh F, McNutt MA: Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol 123: 195–203, 1986
Key G, Becker MH, Baron B, Duchrow M, Schluter C, Flad HD, Gerdes J: New Ki-67–equivalent marine monoclonal antibodies (MIB-1–3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest 68: 629–636, 1993
Vanden Berg FM, Baas IO, Polak MM, Offenhaus GJA: Detection of p53 overexpression in routinaly paraffinembedded tissue of human carcinomas using a novel target unmasking fluid. Am J Pathol 142: 381–385, 1993
Sato Y, Nakajima T, Kuroki M, Abe Y, Watanabe S: Examination of the unlabelled antibody enzyme (PAP) method. Rinsho-Kensa 25: 449–453, 1981 (in Japanese)
Russel DS, Rubinstein LJ: Pathology of tumors of the Nervous system. 5th ed.,Williams and Wilkins, Baltimore, 1989, 792 pp
Fuller GN, Burger PC: Central nervous system. In: Stemberg SS (ed) Histology for Pathologists. Raven Press, New York, 1992, pp 145–167.
Lewis MG: Melanoma and pigmentation of the leptomeninges in Ugandan Africans. J Clin Pathol 22: 183–186, 1969
Quaba AA, Wallace AF: The incidence of malignant melanoma (0 to 15 years of age) arising in 'large' congenital nevocellular nevi. Plast Reconstr Surg 78: 174–179, 1986
Ruiz-Maldonado R, Tamayo L, Laterzo AM, Duran C: Giant pigmented nevi: Clinical, histopathologic, and therapeutic considerations. J Pediatr 120: 906–911, 1992
Slaughter JC, Hardman JM, Kempe LG, Earle KM: Neurocutaneous melanosis and leptomeningeal melanomatosis in children. Arch Pathol 88: 298–304, 1969
Boni R, Doguoglu A, Burg G, Muller B, Dummer R: MIB-1 immunoreactivity correlates with metastatic dissemination in primary thick cutaneous melanoma. J AmAcad Dermatol 35: 416–418, 1996
Ramsay JA, From L, Iscoe NA, Kahn HJ: MIB-1 proliferative activity is a significant prognostic factor in primary thick cutaneous melanomas. J Invest Dermatol 105: 22–26, 1995
Berger DP, Herbstritt L, Dengler WA, Marme D, Mertelsmann R, Fiebig HH: Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Ann Oncol 6: 817–825, 1995
Halaban R: Growth factors and melanomas. Seminars in Oncology 23: 673–681, 1996
Lubbe J, Reichel M, Burg G, Kleihues P: Absence of p53 gene mutations in cutaneous melanoma. J Invest Dermatol 102: 819–821, 1994
Poremba C, Yandell DW, Metze D, Kamanabrou D, Bocker W, Dockhorn-Dworniczak B: Immunohistochemical detection of p53 in melanomas with rare p53 gene mutations is associated with mdm-2 overexpression. Oncol Res 7: 331–339, 1995
Saenz-Santamaria MC, McNutt NS, Bogdany JK, Shea CR: p53 expression is rare in cutaneous melanomas. Am J Dermatol 17: 344–349, 1995
Cordon-Cardo C, Dalbagni G, Saez GT, Oliva MR, Zhang Z-F, Rosai J, Reuter VE, Pellicer A: p53 mutations in human bladder cancer: Genotypic versus phenotypic patterns. Int J Cancer 56: 347–353, 1994
Esrig D, Spruck CH III, Nichols PW, Chaiwun B, Steven K, Groshen S, Chen SC, Skinner DG, Jones PA, Cote RT: p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol 143: 1389–1397, 1993
Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, Hirohashi S: Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol 142: 1534–1543, 1993
Kennedy SM, MacGeogh C, Jaffe R, Spurr NK: Overexpression of the oncoprotein p53 in primary hepatic tumors of childhood does not correlate with gene mutations. Hum Pathol 25: 438–442, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oka, H., Kameya, T., Hata, T. et al. Leptomeningeal Melanomatosis With Multiple Cutaneous Pigmented Nevi: Tumor Cell Proliferation and Malignant Transformation in an Autopsy Case. J Neurooncol 44, 41–45 (1999). https://doi.org/10.1023/A:1006127619926
Issue Date:
DOI: https://doi.org/10.1023/A:1006127619926