Abstract
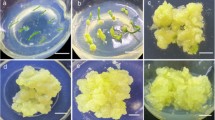
Clonal micropropagation of Jerusalem artichoke (Helianthus tuberosus L.) was initiated from axillary meristems of lateral shoots of field-grown plants on medium with MS salts, 2% sucrose, 1 mg l-1 thiamine-HCl, 1 mg l-1 IAA and 0.6% agar. Plantlets were cut into nodal sections and used for subsequent subcultures and for microtuber induction. Microtubers were induced from axillary meristems on medium with half-strength MS salts, 8% sucrose and 0.5 mg l-1 BA in darkness at 18 °C. They had near to 30% of dry matter. Microtubers resumed growth in light room at 23 °C after 4–6 months of cold storage.
Similar content being viewed by others
References
Bourque JE, Miller JC & Park WD (1987) Use of an in vitro tuberization system to study tuber protein gene expression. In Vitro Cell. Dev. Biol. 23: 381–386
Dodds JH, Silva-Rodrigues D & Tovar P (1992) Micropropagation of potato (Solanum tuberosum L.). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, Vol 19 (pp 91–106). Springer Verlag, Berlin
Esan EB (1992) Micropropagation of cocoa (Theobroma cacao L.)In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, Vol 19 (pp 91–106). Springer Verlag, Berlin
Ewing EE (1985) Cuttings as simplified models of potato plant. In: Li PH (ed) Potato Physiology (pp 153–207). Academic Press, Orlando
Garner N & Blake J (1989) The induction and development of potato microtubers in vitro on media free of growth regulating substances. Ann. Bot. 63: 663–674
Grassert V, Pett B & Thieme R (1986) Investigations into the chemical composition of microtubers derived from potato in vitro plants. Arch. Zuchtungsforsch. 16: 175–178
John P (1992) Biosynthesis of the Major Crop Products. Wiley & Sons, Chichester Koda Y (1992) The role of jasmonic acid and related compounds in the regulation of plant development. Int. Rev. Cytol. 135: 155–199
Koda Y & Kikuta Y (1991) Possible involvement of jasmonic acid in tuberization of yam plants. Plant Cell Physiol. 32: 629–633
Koda Y & Okazawa Y (1988) Detection of potato tuber-inducing activity in potato leaves and old tubers. Plant Cell Physiol. 29: 969–974
Melis RJM & van Staden (1984) Tuberization and hormones. Z. Pflanzenphysiol. 113: 271–283
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Ng SYC (1988) In vitro tuberization in white yam (Dioscorea rotundata Poir). Plant Cell Tiss. Org. Cult. 14: 121–128
Ng SYC (1992) Micropropagation of white yam (Dioscorea rotundata Poir.). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, Vol. 19 (pp 135–159). Springer Verlag, Berlin
Nowak J & Colborne D (1989) In vitro tuberization and proteins as indicators of heat stress tolerance in potato. Amer. Potato J. 66: 35–45
Vreugdenhil D & Struik PC (1989) An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiol. Plant. 75: 525–531
Wissmann AM & Tripathi BK (1977) Recherches sur la tuberization de fragments de tiges de topinambour cultives in vitro. C. R. Acad. Sci. (Paris) D285: 1431–1433
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gamburg, K.Z., Vysotskaya, E. & Gamanets, L. Microtuber formation in micropropagated Jerusalem artichoke (Helianthus tuberosus). Plant Cell, Tissue and Organ Culture 55, 115–118 (1998). https://doi.org/10.1023/A:1006127319351
Issue Date:
DOI: https://doi.org/10.1023/A:1006127319351