Abstract
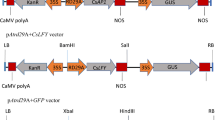
We have previously identified two cDNAs encoding vegetative storage proteins (VSPs) in Arabidopsis thaliana. Unlike soybean in which VSPs accumulate at high levels in leaves, A. thaliana VSP mRNAs are abundant in flowers. To understand tissue-specific expression and possible roles of VSPs on reproductive organ development, genes corresponding to VSPs (Vsp1 and Vsp2) and their putative promoters were characterized in this study. Genomic sequence analysis revealed that Vsp1 and Vsp2 resemble each other except in their introns, and that these two genes were organized in a tandem array with an interval of 6 kb in a region. The expression patterns of Vsp1 and Vsp2 were examined using transgenic A. thaliana plants carrying a promoter from Vsp1 or Vsp2 fused to a bacterial β-glucuronidase (GUS) reporter gene. The promoter from Vsp1 expressed its effect in gynoecia, especially in styles, the basal and distal ends of ovaries and in siliques, whereas the promoter from Vsp2 showed its activity in vegetative shoots, petioles, peduncles and receptacles of floral organs. These results suggest that expression of Vsp1 and Vsp2 may be developmentally regulated in A. thaliana. In the transgenic plants, the GUS activity was induced by wounding in an area around the mid-rib of leaves. Therefore, Vsp1 and Vsp2 promoters appear to have elements required for both tissue specificity and wounding.
Similar content being viewed by others
References
Bartel B, Fink GR: Differential regulation of an auxin-producing nitrilase gene family in Arabidopsis thaliana. Proc Natl Acad Sci USA 91: 6649–6653 (1994).
Bender J, Fink GR: Epigenetic control of an endogenous gene family is revealed by a novel blue fluorescent mutant of Arabidopsis. Cell 83: 725–734 (1995).
Benedetti CE, Xie D, Turner JG: COI1-dependent expression of an Arabidopsis vegetative storage protein in flowers and siliques and in response to coronatine or methyl jasmonate. Plant Physiol 109: 567–572 (1995).
Berger S, Bell E, Sadka A, Mullet JE: Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB, gene encoding vegetative storage protein acid phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol 27: 933–942 (1995).
Creelman RA, Tierney ML, Mullet JE: Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89: 4938–4941 (1992).
Deblaere R, Bytebier B, De Grave H, Deboeck F, Sehell J, Van Montagu M, Leemans J: Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucl Acids Res 13: 4777–4788 (1985).
DeWald DB, Mason HS, Mullet JE: The soybean vegetative storage proteins VSP α and VSP β are acid phosphatases active on polyphosphates. J Biol Chem 267: 15958–15964 (1992).
Doyle JJ, Doyle JL, Hortorium LHB: Isolation of plant DNA from fresh tissue. Focus 12: 13–15 (1990).
Feys BJ, Benedetti CE, Penfold CN, Turner JG: Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 (1994).
Franceschi VR, Wittenbach VA, Giaquinta RT: Paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. Plant Physiol 72: 586–589 (1983).
Ito T, Hirano M, Akama K, Shimura Y, Okada K: Touchinducible genes for calmodulin and a calmodulin-related protein are located in tandem on a chromosome of Arabidopsis thaliana. Plant Cell Physiol 36: 1369–1373 (1995).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Kim SR, Choi JL, Costa MA, An G: Identification of G-box sequence as essential element for methyl jasmonate response of potato proteinase inhibitor II promoter. Plant Physiol 99: 627–631 (1992).
Logemann J, Schell J, Willmitzer L: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Mason HS, Dewald DB, Mullet JE: Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell 5: 241–251 (1993).
Mason HS, Guerrero FD, Boyer JS, Mullet JE: Proteins homologous to leaf glycoproteins are abundant in stems of dark-grown soybean seedlings. Analysis of proteins and cDNAs. Plant Mol Biol 11: 845–856 (1988).
Mason HS, Mullet JE: Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell 2: 569–579 (1990).
Mueller PR, Wold B: In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science 246: 780–786 (1989).
Rapp WD, Lilley GG, Nielsen NC: Characterization of soybean vegetative storage proteins and genes. Theor Appl Genet 79: 785–792 (1990).
Ryan CA: The search for the proteinase inhibitor-inducing factor, PIIF. Plant Mol Biol 19: 123–133 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Spilatro SR, Anderson JM: Characterization of a soybean leaf protein that is related to the seed lectin and is increased with pod removal. Plant Physiol 90: 1387–1393 (1989).
Staswick PE: Soybean vegetative storage protein structure and gene expression. Plant Physiol 87: 250–254 (1988).
Staswick PE: Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol 89: 309–315 (1989).
Staswick PE: Novel Regulation of vegetative storage protein genes. Plant Cell 2: 1–6 (1990).
Staswick PE: Storage protein of vegetative plant tissues. Annu Rev Plant Physiol 45: 303–322 (1994).
Utsugi S, Sakamoto W, Ogura Y, Murata M, Motoyoshi F: Isolation and characterization of cDNA clones corresponding to the genes expressed preferentially in floral organs of Arabidopsis thaliana. Plant Mol Biol 32: 759–765 (1996).
Van Lijswbettens M, Van Derhaeghen R, Van Montagu M: Insertional mutagenesis in Arabidopsis thaliana: isolation of a T-DNA-linked mutation that alters leaf morphology. Theor Appl Genet 81: 277–284 (1991).
Williams ME, Foster R, Chua NH: Sequences flanking the hexameric G-box core CACGTG affect the specificity of protein binding. Plant Cell 4: 485–496 (1992).
Wittenbach VA: Effect of pod removal on leaf senescence in soybeans. Plant Physiol 70: 1544–1548 (1982).
Wittenbach VA: Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol 73: 121–124 (1983).
Xue J, Jorgensen M, Pihlgren U, Rask L: The myrosinase gene family in Arabidopsis thaliana: gene organization, expression and evolution. Plant Mol Biol 27: 911–922 (1995).
Yanisch-Perron C, Vieira J, Messing J: Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–319 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Utsugi, S., Sakamoto, W., Murata, M. et al. Arabidopsis thaliana vegetative storage protein (VSP) genes: gene organization and tissue-specific expression. Plant Mol Biol 38, 565–576 (1998). https://doi.org/10.1023/A:1006072014605
Issue Date:
DOI: https://doi.org/10.1023/A:1006072014605