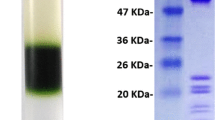
Abstract
Photosystem I (PS I) complexes from two strains of the marine photosynthetic prokaryote Prochlorococcus, MED4 (= clone CCMP1378) and SS120 (= clone CCMP1375), were isolated by centrifugation on sucrose gradients after detergent treatment. The PS I-enriched fractions of both strains contained about 100 chlorophyll molecules per P700. Electron microscopy showed that the PS I complexes were in a trimeric form. The characteristic long wavelength fluorescence emission of PS I at 77 K, currently observed in chloroplasts and most cyanobacteria was absent both in intact cells and in PS I preparations of both strains. The major proteins of the PS I-enriched fractions were identified immunologically as PsaA and PsaB. Two proteins with apparent molecular masses of about 21 and 25 kDa were present in PS I preparations of Prochlorococcus, whereas the small PS I subunits in cyanobacteria all have molecular masses below 18 kDa. The 25 kDa protein showed a strong cross-reaction with a heterologous antibody against PsaL. Relatedness of the 21 kDa protein to PsaF was demonstrated by internal protein sequencing. Although only trace amounts of the major divinyl-Chl a/b-binding antenna complexes were present in the PS I preparations, significant amounts of divinyl-Chl b were observed in this fraction. The putative organization of this Chl b in PS I is discussed.
Similar content being viewed by others
References
Allen KD and Staehelin LA (1991) Resolution of 16 to 20 chlorophyll-protein complexes using a low ionic strength native green gel system. Anal Biochem 194: 214–222
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15
Bengis C and Nelson N (1975) Purification and properties of the Photosystem I reaction center from chloroplasts. J Biol Chem 250: 2783–2788
Boekema EJ, Boonstra AF, Dekker JP and Rögner M (1994) Electron microscopic structural analysis of Photosystem I, Photosystem II, and the cytochrome b 6 /f complex from green plants and cyanobacteria. J Bioenerg Biomembr 26: 17–29
Burger-Wiersma T, Veenhuis M, Korthals HJ, Van de Wiel CCM and Mur LR (1986) A new prokaryote containing chlorophylls a and b. Nature London 320: 262–264
Chisholm SW, Olson RJ, Zettler ER, Goericke R, Waterbury JB and Welschmeyer NA (1988) A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature 334: 340–343
Chisholm SW, Frankel SL, Goericke R, Olson RJ, Palenik B, Waterbury JB, West-Johnsrud L and Zettler ER (1992) Prochlorococcus marinus nov. gen. nov. sp.: An oxyphototrophic marine prokaryote containing divinyl-chlorophyll a and b. Arch Microbiol 157: 297–300
Chitnis VP and Chitnis PR (1993) PsaL subunit is required for the formation of Photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett 336: 330–334
Chitnis PR and Nelson N (1992) Assembly of two subunits of the cyanobacterial Photosystem I on the n-side of thylakoid membranes. Plant Physiol 99: 239–246
Chitnis PR, Xu Q, Chitnis VP and Nechushtai R (1995) Function and organization of Photosystem I polypeptides. Photosynth Res 44: 23–40
Delphin E, Duval JC, Etienne AL and Kirilovsky D (1996) State transitions or ΔpH-dependent quenching of Photosystem II fluorescence in red algae. Biochemistry 35: 9435–9445
Falk S, Samson G, Bruce D, Huner NPA and Laudenbach DE (1995) Functional analysis of the iron-stress induced CP 43' polypeptide of PS II in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res 45: 51–60
Fling SP and Gregerson DS (1986) Peptide and protein molecular mass determination by electrophoresis using a high-molarity Tris buffer system without urea. Anal Biochem 155: 83–88
Goericke R and Repeta DJ (1992) The pigments of Prochlorococcus marinus: The presence of divinyl chlorophyll a and b in a marine prochlorophyte. Limnol Oceanogr 37: 425–433
Golbeck JH (1994) Photosystem I in cyanobacteria In: Bryant DA (ed) The Molecular Biology of Cyanobacteria, pp 319–360. Kluwer Academic Publishers, Dordrecht, The Netherlands
Green BR and Durnford DG (1996) The Chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47: 685–714
Hess WR, Partensky F, van der Staay GWM, Garcia-Fernandez J, Börner T and Vaulot D (1996) Coexistence of phycoerythrin and a chlorophyll a/b antenna in a marine prokaryote. Proc Natl Acad Sci USA 93: 11126–11130
Hiller RG and Larkum AWD (1985) The chlorophyll-protein complexes of Prochloron sp. (Prochlorophyta). Biochim Biophys Acta 806: 107–115
Hiyama T and Ke B (1972) Difference spectra and extinction coefficient of P700. Biochim Biophys Acta 267: 160–171
Kowallik KV, Stoebe B, Schaffran I, Kroth-Pancic P and Freier U (1995) The chloroplast genome of a chlorophyll a+c-containing alga, Odontella sinensis. Plant Mol Biol Rep 13: 336–342
Kraay GW, Zapata M and Veldhuis MJW (1992) Separation of chlorophylls c 1, c 2, c 3 of marine phytoplankton by reversedphase C18 high performance liquid chromatography. J Phycol 28: 708–712
Krauß N, Schubert WD, Klukas O, Fromme P, Witt HT and Saenger W (1996) Photosystem I at 4. A resolution represents the first structural model of joint photosynthetic reaction center and core antenna system. Nature Struct Biol 3: 965–973
Krause GH and Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Ann Rev Plant Physiol Plant Mol Biol 42: 313–349
Kruip J, Chitnis PR, Lagoutte B, Rögner M and Boekema J (1997) Structural organization of the major subunits in cyanobacterial Photosystem I. J Biol Chem 272: 17061–17069
Larkum AWD, Scaramuzzi C, Cox GC, Hiller RG and Turner AG (1994) Light-harvesting chlorophyll c-like pigment in Prochloron. Proc Natl Acad Sci USA 91: 679–683
LaRoche J, van der Staay GWM, Partensky F, Aebersold R, Ducret A, Li R, Golden SS, Hiller RG, Larkum AWD, Wrench PM and Green BR (1996) Independent evolution of the chlorophyll a/b light-harvesting proteins of prochlorophytes and higher plants. Proc Nat Acad Sci USA 93: 15244–15248
Laudenbach DE and Straus NA (1988) Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J Bacteriol 170: 5018–5026.
Lewin RA (1976) Prochlorophyta as a proposed new division of algae. Nature London 261: 697–698
Li N, Zhao J, Warren JT, Warden JT, Bryant DA and Golbeck JH (1991) PsaD is required for the stable binding of PsaC to Photosystem I core protein of Synechococcus sp. PCC 6301. Biochemistry 30: 7863–7872
Lichtlé C, Thomas JC, Spilar A and Partensky F (1995) Immunological and ultrastructural characterization of the photosynthetic complexes of the Prochlorophyte Prochlorococcus (Oxychlorobacteria). J Phycol 31: 934–941
Marquardt J, Senger H, Miyashita H, Miyachi S and Mörschel E (1997). Isolation and characterization of biliprotein aggregates from Acaryochloris marina, a Prochloron-like prokaryote containing mainly chlorophyll d. FEBS Lett 410: 428–432
Matthijs HCP, van der Staay GWM and Mur LR (1994) Prochlorophytes: the 'other' cyanobacteria? In: Bryant DA (ed) TheMolecular Biology of Cyanobacteria, pp 49–64. Kluwer Academic Publishers, Dordrecht, The Netherlands
Miyashita H, Adachi K, Kurano N, Ikemoto H, Chiara M and Miyachi S (1997) Pigment composition of a novel oxygenic photosynthetic prokaryote containing Chl d as the major chlorophyll. Plant Cell Physiol 38: 274–281.
Moore LR, Goericke R and Chisholm SW (1995) Comparative physiology of Synechococcus and Prochlorococcus: Influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar Ecol Prog Ser 116: 259–275
Newman PJ and Sherman LA (1978) Isolation and characterization of Photosystem I and II membrane particles from the blue alga, Synechococcus cedrorum. Biochim Biophys Acta 5023: 343–361
Partensky F, Blanchot J, Lantoine F, Neveux J and Marie D (1996) Vertical structure of picophytoplankton at different trophic sites of the tropical northeastern Atlantic Ocean. Deep Sea Res I 43: 1191–1213
Partensky F, La Roche J, Wyman K and Falkowski PG (1997) The divinyl-chlorophyll a/b complexes of two strains of the oxyphototrophic marine prokaryote Prochlorococcus: Characterization and response to changes in growth irradiance. Photosynth Res 51: 209–222.
Rögner M, Nixon PJ and Diner AD (1989) Purification and characterization of Photosystem I and II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem 265: 6189–6196
Scanlan DJ, Hess WR, Partensky F, Scanlan J and Vaulot D (1996) High degree of genetic variation in Prochlorococcus (Prochlorophyta) revealed by RFLP analysis. Eur J Phycol 31: 1–9
Schuster G, Nechushtai R, Nelson BL and Ohad I (1985) Purification and composition of Photosystem I reaction center of Prochloron sp., an oxygen-evolving prokaryote containing chlorophyll b. FEBS Lett 191: 29–33
Schuster G, Owens GC, Cohen Y and Ohad I (1984) Thylakoid polypeptide composition and light-independent phosphorylation of the chlorophyll a, b-protein in Prochloron, a prokaryote exhibiting oxygenic photosynthesis. Biochim Biophys Acta 767: 596–605
Siefermann-Harms D (1985) Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim Biophys Acta 811: 325–355
Towbin SE, Staehelin T and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Urbach E, Scanlan DJ, Distel DL, Waterbury JB and Chisholm SW (1998) Rapid diversification of marine picophytoplankton with dissimilar light-harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (Cyanobacteria). J Mol Evol 46: 188–201
van der Staay GWM, Brouwer A, Baard RL, van Mourik F and Matthijs HCP (1992) Separation of photosystems I and II from the oxychlorobacterium (prochlorophyte) Prochlorothrix hollandica and association of chlorophyll b binding antennae with Photosystem II. Biochim Biophys Acta 1102: 220–228
van der Staay GWM and Staehelin LA (1994) Biochemical characterization of protein composition and protein phosphorylation patterns in stacked and unstacked thylakoid membranes of the prochlorophyte Prochlorothrix hollandica. J Biol Chem 269: 24834–24844
Xu Q, Armbrust TS, Guikema JA and Chitnis PR (1994) Organization of Photosystem I polypeptides: A structural interaction between PsaD and PsaL subunits. Plant Physiol 106: 1057–1063.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garczarek, L., van der Staay, G.W.M., Thomas, J.C. et al. Isolation and characterization of Photosystem I from two strains of the marine oxychlorobacterium Prochlorococcus. Photosynthesis Research 56, 131–141 (1998). https://doi.org/10.1023/A:1006049832657
Issue Date:
DOI: https://doi.org/10.1023/A:1006049832657