Abstract
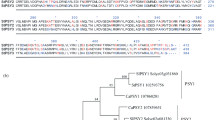
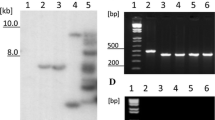
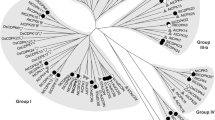
Prosystemin is the precursor protein of the 18 amino acid wound signal systemin which activates systemic defense in tomato leaves against insect herbivores (McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA, Science 255 (1992) 1570–1573). Here, we report the isolation of cDNA sequences encoding prosystemin from potato (Solanum tuberosum), black nightshade (S. nigrum), and bell pepper (Capsicum annuum), all members of the Solanaceae family, using reverse-transcription polymerase chain reaction (RT-PCR). Pairwise comparisons of the predicted prosystemin proteins from the three species with tomato prosystemin and among each other indicated sequence identities ranging from 73% to 88%. The deduced systemin polypeptides were synthesized and tested for their capacities to induce the synthesis of the defensive proteinase inhibitors in tomato leaves. Potato and pepper systemins were approximately as active as tomato systemin, whereas nightshade systemin was ten-fold less active. The accumulation of proteinase inhibitor mRNA transcripts could be induced in each of these plants by treatment with the homologous systemin. As in the tomato, in potato, black nightshade, and bell pepper plants, prosystemin homologs appear to function as precursors of systemic wound signals.
Similar content being viewed by others
References
Becraft PW, Stinard PS, McCarty DR: CRINKLY4: A TNFRlike receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409 (1996).
Bergey DR, Howe GA, Ryan CA: Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA 93: 12053–12058 (1997).
Beuning LL, Spriggs TW, Christeller JT: Evolution of the proteinase inhibitor I family and apparent lack of hypervariability in the proteinase contact loop. J Mol Evol 39: 644–654 (1994).
Borson ND, Salo WL, Drewes LR: A lock-docking oligo(dt) primer for 5′ and 3′ RACE PCR. PCR Meth Appl 2: 144–148 (1992)
Bradshaw Jr H, Hollick JB, Parsons TJ, Clarke HRG, Gordon MP: Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol 14: 51–59 (1989).
Brown WE, Ryan CA: Isolation and characterization of a wound-induced trypsin inhibitor from alfalfa leaves. Biochemistry 23: 3418–3422 (1984).
Cleveland TE, Thornburg RW, Ryan CA: Molecular characterization of a wound-inducible inhibitor I gene from potato and the processing of its mRNA and protein. Plant Mol Biol 8: 199–207 (1985).
Constabel CP, Bergey DR, Ryan CA: Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci USA 92: 407–411 (1995).
Frohman M: RACE: Rapid amplification of cDNA ends. In: Innis MA (ed) PCR Protocols: AGuide to Methods and Applications, pp. 28–37. Academic Press, San Diego, CA (1990).
Graham JS, Hall G, Pearce G, Ryan CA: Regulation of synthesis of proteinase inhibitor I and II mRNAs in leaves of wounded tomato plants. Planta 169: 399–405 (1986).
Graham JS, Pearce G, Merryweather J, Titani K, Ericsson L, Ryan CA: Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of preinhibitor I and its post-translational processing. J Biol Chem 260: 6555–6560 (1985).
Graham JS, Pearce G, Merryweather J, Titani K, Ericsson LH, Ryan CA: Wound-induced proteinase inhibitors from tomato leaves II: The cDNA-deduced primary structure of preinhibitor. II. J Biol Chem 260: 6561–6564 (1985).
Green TR, Ryan CA: Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175: 776–777 (1972).
Hummel B: A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 37: 1393–1399 (1959).
Marchuk D, Dumm M, Saulino A, Collins FS: Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucl Acids Res 19: 1154 (1991).
McGurl B, Ryan CA: The organization of the prosystemin gene. Plant Mol Biol 20: 405–409 (1992).
McGurl B, Orozco-Cardenas M, Pearce G, Ryan CA: Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci USA 91: 9799–9802 (1994).
McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA: Structure, expression, and antisense inhibition of the systemin precursor gene. Science 255: 1570–1573 (1992).
Pearce G, Johnson S, Ryan CA: Purification and characterization from tobacco (Nicotiana tabacum) leaves of six small, wound-inducible proteinase isoinhibitors of the potato inhibitor II family. Plant Physiol 102: 639–644 (1993).
Pearce G, Johnson S, Ryan CA: Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem 268: 212–216 (1993).
Pearce G, Strydom D, Johnson S, Ryan CA: A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895–898 (1991).
Peña-Cortes H, Sanchez-Serrano J, Rocha-Rosa M, Willmitzer L: Systemic induction of proteinase-inhibitor-II gene expression in potato plants by wounding. Planta 174: 84–89 (1988).
Program Manual for theWisconsin Package, Version 8. Genetics Computer Group Inc., Madison, WI (1994).
Realini C, Rogers SW, Rechsteiner M: Proposed roles in protein-protein association and presentation of peptides by the MHC class I receptors. FEBS Lett 348: 109–113 (1994).
Rohrmeier T, Lehle L: WIP1, a wound-inducible gene from maize with homology to Bowman-Birk proteinase inhibitors. Plant Mol Biol 22: 783–792 (1993).
Ryan CA: Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopath 28: 425–429 (1990).
Ryan CA: Quantitative determination of soluble cellular proteins by radial diffusion in agar gel containing antibodies. Anal Biochem 19: 434–440 (1967).
Ryan CA: The search for the proteinase inhibitor-inducing factor, PIIF. Plant Mol Biol 19: 123–133 (1992).
Saarikoski P, Clapham D, von Arnold S: A wound-inducible gene from Salix viminalis coding for a trypsin inhibitor. Plant Mol Biol 31: 465–478 (1996).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Schaller A, Ryan CA: Systemin: a polypeptide defense signal in plants. BioEssays 18: 27–33 (1995).
Seldal T, Andersen K-J, Högstedt G: Grazing-induced proteinase inhibitors: a possible cause for lemming population cycles. Oikos 70: 3–11 (1994).
Trautman R, Cowan KM, Wagner GG: Data for processing for radial immunodiffusion. Immunochemistry 8: 901–916 (1971).
van de Sande K, Pawlowski K, Czaja I, Wieneke U, Schell J, Schmidt J, Walden R, Matvienko M, Wellink J, van Kammen A, Franssen H, Bisseling T: Modification of phytohormone response by a peptide encoded by ENOD40 of legumes and a nonlegume. Science 273: 370–373 (1996).
Walker-Simmons M, Ryan CA: Wound-induced accumulation of trypsin inhibitor activities in plant leaves. Plant Physiol 59: 437–439 (1977).
Wingate VPM, Broadway RM, Ryan CA: Isolation and characterization of a novel, developmentally regulated proteinase inhibitor I protein and cDNA from the fruit of a wild tomato species. J Biol Chem 264: 17734–17738 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Constabel, C.P., Yip, L. & Ryan, C.A. Prosystemin from potato, black nightshade, and bell pepper: primary structure and biological activity of predicted systemin polypeptides. Plant Mol Biol 36, 55–62 (1998). https://doi.org/10.1023/A:1005986004615
Issue Date:
DOI: https://doi.org/10.1023/A:1005986004615