Abstract
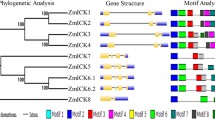
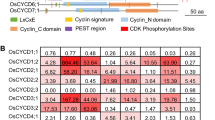
Cyclins are involved in the regulation of cell cycle progression in eukaryotes. We have isolated a cyclin cDNA clone, cycZm2w, from maize root tip cells, which fits best into group A2 of current plant cyclin gene classification schemes. The cDNA encodes a protein with a domain homologous to the cyclin box of mitotic cyclins. Complementation studies revealed that cycZm2w was able to rescue a budding yeast cyclin-deficient mutant (BF305–15d#21). As expected, cycZm2w is expressed in organs of the maize plant that possess meristematic activity, but is especially prominent in the proliferating regions of the root apex.
Similar content being viewed by others
References
Barlow P: Regeneration of the cap of primary roots of Zea mays. New Phytol 73: 937–954 (1974).
Becker DM, Guarente L: High efficiency transformation of yeast by electroporation. Meth Enzymol 194: 182–187 (1991).
Beelman CA, Parker R: Degradation of mRNA in eukaryotes. Cell 81: 79–183 (1995).
Booher RN, Beach, DH: Involvement of cdc13 + in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J 7: 2321–2327 (1988).
Chen DC, Yan BC, Kuo TT: One step transformation of yeast in stationary phase. Curr Genet 21: 83–84 (1992).
Colicelli J, Birchmeier C, Michaeli T, O'Neill K, Riggs M, Wigler M: Isolation and characterization of a mammalian gene encoding a highaffinity cAMP phosphodiesterase. Proc Natl Acad Sci USA 86: 3599-3603 (1989).
Day IS, Reddy ASN: Cloning of a family of cyclins from Arabidopsis thaliana. Biochim Biophys Acta 1218: 115–118 (1994).
Dellaporta SL, Wood J, Hicks JB: A plant DNA minipreparation version II. Plant Mol Biol Rep 1: 19–21 (1983).
Evans, T, Rosenthal ET, Youngblom J, Distel D, Hunt T: Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33: 389–396 (1983).
Feinberg AP, Vogelstein B: Atechnique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Feldman LJ: The de novo origin of the quiescent center in regenerating root apices of Zea mays. Planta 128: 207–212 (1976).
Ferreira, PCG, Hemerly AS, Villarroel R, Van Montagu M, Inze D: Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6: 1763–1774 (1994).
Ferreira, PCG, Hemerly AS, deAlmeida Engler J, Bergounioux C, Burssens S, Van Montagu M, Eugler G, Inzé D: Three discrete classes of Arabidopsis cyclins are expressed during different intervals of the cell cycle. Proc Natl Acad Sci USA 91: 11313–11317 (1994).
Fobert PR, Coen ES, Murphy GJP, Doonan JH: Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J 13: 616–624 (1994).
Fuerst RAUA., Soni R, Murray JAH, Lindsey K: Modulation of cyclin transcript levels in cultured cells of Arabidopsis thaliana. Plant Physiol 112: 1023–1033 (1996).
Gautier J, Norbury C, Lohka M, Nurse P, Maller J: Purified maturation promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell 54: 433–439 (1989).
Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL: Cyclin is a component of maturationpromoting factor from Xenopus. Cell 60: 487–494 (1990).
Glotzer M, Murray AW, Kirschner MW: Cyclin is degraded by the ubiquitin pathway. Nature 349: 132–138 (1991).
Hartwell LH, Weinert TA: Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634 (1989).
Hata S, Kouchi H, Suzuka I, Ishii T: Isolation and characterization of cDNA clones for plant cyclins. EMBO J 10: 2681–2688 (1991).
Hemerly A, Bergounioux C, Van Montagu M, Inzé D, Ferreira P: Genes regulating the plant cell cycle: isolation of a mitoticlike cyclin from Arabidopsis thaliana. Proc NatlAcad Sci USA 89: 3295–3299 (1992).
Hirt, H, Mink M, Pfosser M, Bogre L, Gyorgyey J, Jonak C, Gartner A, Dudits D, HeberleBors E: Alfalfa cyclins: differential expression during the cell cycle and in plant organs. Plant Cell 4: 1531–1538 (1992).
Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP: Mechanism of CDK activation revealed by the structure of a cyclinACDK2 complex. Nature 376: 313–320 (1995).
Knoblich JA, Lechner CF: Synergistic action of Drosophila cyclins A and B during the G2M transition. EMBO J 12: 65–74 (1993).
Kouchi H, Sekine M, Hata S: Distinct classes ofmitotic cyclins are differentially expressed in the soybean shoot apex during the cell cycle. Plant cell 7: 1143–1155 (1995).
Kreutzer MA, Richards JP, De SilvaUdawatta MN, Temenak JJ, Knoblich JA, Lehner CF, Bennett KL, Caenorhabdites elegans cyclin Aand Btype genes: a cyclin A multigene family, an ancestral cyclin B3 and differential germline expression. J Cell Sci 108: 2415–2424 (1995).
Kuhne C, Linder, P: A new pair of Btype cyclins from Saccharomyces cerevisiae that function early in the cell cycle. EMBO J 12: 3437–3447 (1993).
Kuntzel H., Rottjakob H, Schwed A, Zwerschke W: START control in cycling Saccharomyces cerevisiae cells. Prog Nucl Acids Res Mol Biol 48: 1–28 (1994).
Lew DJ, Dulic V, Reed SI: Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66: 1197–1206 (1991).
Leopold P, O'Farrell PH: An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient inG1 cyclins. Cell 66: 1207–1216 (1991).
Lohka MJ, Hayes MK, Maller JL: Purification of maturation promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci USA 85: 3009–3013 (1988).
Meskiene I, Bogre L, Dahl M, Pirck M, Ha DTC, Swoboda I, HeberieBors E, Ammerer G, Hirt H: CycMs3, a novel Btype alfalfa cyclin gene, is induced in the G0toG1 transition of the cell cycle. Plant Cell 7: 759–771 (1995).
Minshull J, Pines J, Golsteyn R, Standart N, Mackie S, Colman A, Blow J, Ruderman JV, Wu M, Hunt T: The role of cyclin synthesis, modification and destruction in the control of cell division. J Cell Sci (suppl.) 12: 77–97 (1989).
Nah R, Tokiwa G, Anand S, Erickson K, Futcher AB. The WH11+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J 7: 4335–4346 (1988).
Nigg EA: Cyclindependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays 17: 471–480 (1995).
Norbury C, Nurse P: Control of the higher eukaryotic cell cycle by p34cdc2 homologs. Biochim Biophys Acta 989: 85–95 (1989).
Qin LX, Richard L, Perennes C, Gadal P, Bergounioux C: Identification of a cell cyclerelated gene, cyclin, in Nicotiana tabacum (L.). Plant Physiol 108: 425–426 (1995).
Reichheld JP, Chaubet N, Shen WH, Renaudin JP, Gigot C: Multiple Atype cyclins express sequentially during the cell cycle oin Nicotiana tabacum BY2 cells. Proc Natl Acad Sci USA 93: 13819–13824 (1996).
Renaudin JP, Colasanti J, Rime H, Yan Z, Sundaresan V: Cloning of four cyclins from maize indicates that higher plants have three structurally, distinct groups of mitotic cyclins. Proc Natl Acad Sci USA 91: 7375–7379 (1994).
Renaudin JP, Doonan JH, Freeman D, Hashimoto J, Hirt H, Inzé D, Jacobs T, Kouchi H, Rouze P, Sauter M, Savoure A, Sorrell DA, Sundaresan V, Murray JAH: Plant cyclins: a unified nomenclature for plant A, Band Dtype cyclins based on sequence organisation. Plant Mol Biol 32: 1003–1018 (1997).
Richardson H, Lew DJ, Henze M, Sugimoto K, Reed SI: Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Devel 6: 2021–2034 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger FS, Nicksen I, Coulson AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sauter M, Mekhedov SL, Kende H: Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J 7: 623–632 (1995).
Savoure A, Feher A, Kalo P, Petrovics G, Csanadi G, Szecsi J, Kiss G, Brown S, Kondorosi A, Kondorosi E: Isolation of a fulllength mitotic cyclin cDNA clone cycIIIMs from Medicago sativa: chromosomal mapping and expression. Plant Mol Biol 27: 1059–1070 (1995).
Setiady YY, Sekine M, Hariguchi N, Yamamoto T, Kouchi H, Shinmyo A: Tobacco mitotic cyclins: cloning, characterization, gene expression and functional assay. Plant J 8: 949–957 (1995).
Soni R, Carmichael JP, Shah ZH, Murray JAH: A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7: 85–103 (1995).
Steeves TA., Sussex IM: Patterns in Plant Development. Cambridge University Press, Cambridge, UK (1989).
Strathern JN, Higgins DR: Recovery of plasmids from yeast into Escherichia coli: shuttle vectors. Meth Enzymol 194: 319–329 (1991).
Swenson KI, Farrell KM, Ruderman JV: The clam embryo protein cyclin Ainduces entry intoMphase and the resumption of meiosis in Xenopus oocytes. Cell 47: 861–870 (1986).
Szarka S, Fitch M, Schaerer S, Moloney M: Classification and expression of a family of cyclin gene homologues in Brassica napus. Plant Mol Biol 27: 263–275 (1995).
Thompson WF, Everett M, Polans NO, Jorgensen RA, Palmer JD: Phytochrome control of RNA levels in developing pea and mung bean leaves. Planta 158: 487–500 (1983).
Tyers M, Tokiwa G, Futcher B: Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be and upstream activator of Cln1, Cln2 and other cyclins. EMBO J 12: 1955–1968 (1993).
Xiong Y, Connolly T, Futcher B, Beach D: Human Dtype cyclin. Cell 65: 691–699 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hsieh, WL., Wolniak, S.M. Isolation and characterization of a functional A-type cyclin from maize. Plant Mol Biol 37, 121–129 (1998). https://doi.org/10.1023/A:1005968901298
Issue Date:
DOI: https://doi.org/10.1023/A:1005968901298