Abstract
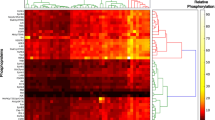
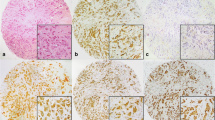
The expression of several apoptosis-regulating genes was evaluated in 9 human breast cancer cell lines, 2 immortalized human mammary epithelial lines, 1 normal breast tissue biopsy, and 3 primary breast tumors, using a multiple antigen detection (MAD) immunoblotting method. The anti-apoptotic proteins Bcl-2, Bcl-XL, Mcl-1, and BAG-1 were present at immunodetectable levels in 7, 10, 10, and 9 of the 11 lines. Comparing these 11 cell lines among themselves revealed that steady-state levels of Bcl-2, Bcl-XL, Mcl-1, and BAG-1 were present at relatively higher levels in 4, 6, 5, and 5 of the lines, respectively. In contrast, the pro-apoptotic proteins Bax and Bak were detected in all 11 cell lines, and were present at relatively higher levels in 10 and 5 of the 11 lines, respectively. The Interleukin-1β converting enzyme (ICE) homolog CPP32 (Caspase-3) was expressed in 10/11 breast cell lines. High levels of p53 protein, indicative of mutant p53, were found in 8 of the 11 lines and correlated inversely with Bax expression (p=0.01). Bcl-2 and BAG-1 protein levels were positively correlated (p = 0.03). Immunoblot analysis of primary adenocarcinomas revealed expression of the anti-apoptotic proteins Bcl-2, Bcl-XL, Mcl-1, and BAG-1, as well as the pro-apoptotic proteins Bax, Bak, and CPP32, in at least 2 of the 3 tumors examined. Immunohistochemical analysis was also performed for all of these proteins using 20 paraffin-embedded breast cancer biopsy specimens that all contained residual normal mammary epithelium in combination with both invasive cancer and carcinoma in situ. All of these apoptosis-regulating proteins were detected in primary breast cancers, though the percentage of immunopositive tumor cells varied widely in some cases. Comparisons of the intensity of immunostaining in normal mammary epithelium and invasive carcinoma suggested that Bcl-2 immunointensity tends to be lower in cancers than normal breast epithelium (p=0.03), whereas CPP32 immunointensity was generally higher in invasive cancers (p < 0.0001). Taken together, the results demonstrate expression of multiple apoptosis-modulating proteins in breast cancer cell lines and primary tumors, suggesting complexity in the regulation of apoptosis in these neoplasms of mammary epithelial origin.
Similar content being viewed by others
References
Nowell PC: The clonal evolution of tumor cell populations. Science 194: 23–28, 1976
Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 124: 1–6, 1994
Reed JC: Bcl-2: Prevention of apoptosis as a mechanism of drug resistance. Hematology/Oncology Clinics of North America 9: 451–474, 1995
Vaux DL, Haecker G, Strasser A: An evolutionary perspective on apoptosis. Cell 76: 777–779, 1994
Reed JC: Regulation of apoptosis by Bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol 7: 541–546, 1995
Patel T, Gores GJ, Kaufmann SH: The role of proteases during apoptosis. FASEB J 10: 587–597, 1996
Steck S, McDonnell T, Sneige N, El-Naggar A: Flow cytometry analysis of apoptosis and Bcl-2 in primary breast carcinomas: clinical and biological implications. Cytometry 24: 116–122, 1996
Neville MC, Daniel CW: The Mammary Gland: Development, Regulation and Function. Plenum Publishing, 1987
Wang TTY, Phang JM: Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res 55: 2487–2489, 1995
Shibata M-A, Maroulakou IG, Jorcyk CL, Gold LG, Ward JM, Green JE: p53-independent apoptosis during mammary tumor progression in C3(1)/SV40 large T antigen transgenic mice: suppression of apoptosis during the transition from preneoplasia to carcinoma. Cancer Res 56: 2998–3003, 1996
Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH: Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Science 109: 631–642, 1996
Wingo PA, Tong T, Bolden S: Cancer Statistics, 1995. CA, Cancer J Clinic 45: 8–30, 1995
Thor AD, Moore DH II, Edgerton SM, Kawasaki ES, Reihsaus E, Kynch HT, Marcus JN, Schwartz L, Chen L-C, Mayall BH, Smith HS: Accumulation of p53 tumor suppressor gene protein: an independent marker of prognosis in breast cancers. J Natl Cancer Inst 84: 845–855, 1992
Miyashita T, Reed JC: Tumor suppressor p53 is a direct transcriptional activator of human bax gene. Cell 80: 293–299, 1995
Krajewski S, Blomvqvist C, Franssila K, Krajewska M, Wasenius V-M, Niskanen E, Reed JC: Reduced expression of pro-apoptotic gene Bax is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res 55: 4471–4478, 1995
Krajewski S, Chatten J, Hanada M, Womer R, Reed JC: Immunohistochemical analysis of the Bcl-2 oncoprotein in human neuroblastoma. Lab Invest 71: 42–54, 1995
Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC: Cloning and functional analysis of BAG-1: a novel Bcl-2 binding protein with anti-cell death activity. Cell 80: 279–284, 1995
Boudreau N, Sympson CJ, Werb Z, Bissell MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267: 891–893, 1995
Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed JC: Immunohistochemical analysis of mcl-1 and bcl-2 proteins in normal and neoplastic lymph nodes. Amer J Pathol 145: 515–525, 1994
Krajewski S, Gascoyne RD, Zapata JM, Krajewska M, Kitada S, Chhanabhai M, Horsman D, Berean K, Piro LD, Fugier-Vivier I, Liu YJ, Wang HG, Reed JC: Immunolocalization of the ICE/Ced-3-family protease, CPP32 (Caspase-3), in non-Hodgkin's lymphomas (NHLs), chronic lymphocytic leukemias (CLL), and reactive lymph nodes. Blood 89: 3817–3825, 1997
Krajewski S, Krajewska M, Reed JC: Immunohistochemical analysis of in vivo patterns of Bak expression, a pro-apoptotic member of the Bcl-2 protein family. Cancer Res 56: 2849–2855, 1996
Krajewski S, Krajewska M, Shabaik A, Wang H-G, Irie S, Fong L, Reed JC: Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 54: 5501–5507, 1994
Reed JC, Yum S, Cuddy MP, Turner BC, Rapp UR: Differential regulation of the p72–74 Raf-1 kinase by RAS and SRC oncoproteins. Cell Growth & Diff 2: 235–243, 1991
Takayama S, Kochel K, Irie S, Inazawa J, Abe T, Sato T, Druck T, Huebner K, Reed JC: Cloning of cDNAs encoding human BAG1 protein and localization of the human BAG1 gene to chromosome 9p12. Genomics 35: 494–498, 1996
Krajewski S, Zapata JM, Reed JC: Detection of multiple antigens on Western blots. Anal Biochem 236: 221–228, 1996
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang H-G, Reed JC: Immunohistochemical determination of in vivo distribution of bax, a dominant inhibitor of bcl-2. Am J Pathol 145: 1323–1333, 1994
Reed JC, Krajewski S, Kitada S, Miyashita T: Methods of measuring Bcl-2 gene expression. In: Herzenberg LA et al (ed) Handbook of Experimental Immunology, 5th edition, Vol. 3, pp 128.1–128.11, 1996
Guan RJ, Moss SF, Arber N, Krajewski S, Reed JC, Holt PR: 30 kDa phosphorylated form of Bcl-2 protein in human colon. Oncogene 12: 2605–2609, 1996
Bartek J, Iggo R, Gannon J, Lane DP: Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 5: 893–899, 1990
Runnebaum IB, Nagarajan M, Bowman M, Soto D, Sukumar S: Mutations in p53 as potential molecular markers for human breast cancer. Proc Natl Acad Sci USA 88: 10657–10661, 1991
Sabourin JC, Martin A, Baruch J, Truc JB, Gompel A, Poitout P: bcl-2 expression in normal breast tissue during the menstrual cycle. Int J Cancer 59: 1–6, 1994
Krajewska M, Wang H-G, Krajewski S, Zapata J, Shabaik A, Gascoyne R, Reed JC: Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res 57: 1605–1613, 1997
Reed JC: Balancing cell life and death: Bax, apoptosis, and breast cancer. J Clin Invest 97: 2403–2404, 1996
Casey G, Lo-Hsueh M, Lopez ME, Vogelstein B, Stanbridge EJ: Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene 6: 1791–1797, 1991
Krajewski S, Thor AD, Edgerton SU, Moore DH, Krajewska N, Reed JC: Immunohistochemical analysis of Bax and Bcl-2 in p53-immunopositive breast cancers. Clin Cancer Res 3: 199–208, 1996
Bargou RC, Daniel PT, Mapara MY, Bommert K, Wagener C, Kallinich B, Royer HD, Dörken B: Expression of the bcl-2 gene family in normal and malignant breast tissue: low bax-??expression in tumor cells correlates with resistance towards apoptosis. Int J Cancer 60: 854–859, 1995
Wagener C, Bargou RC, Daniel PT, Bommert K, Mapara MY, Royer HD, Dörken B: Induction of the death-promoting gene bax-alpha sensitizes cultured breast-cancer cells to drug-induced apoptosis. Int J Cancer 67: 138–141, 1996
Miyashita T, Kitada S, Krajewski S, Horne B, Delia D, Reed JC: Overexpression of the Bcl-2 protein increases the halflife of p21-Bax. J Biol Chem 270: 26049–26052, 1995
Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RJ, Reed JC: BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J in press, 1997
Zeinet M, Gehring U: A protein that interacts with members of the nuclear hormone receptor family: identification and cDNA cloning. Proc Natl Acad Sci USA 92: 11465–11469, 1995
Wang H-G, Takayama S, Rapp UR, Reed JC: Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci USA 93: 7063–7068, 1996
Rights and permissions
About this article
Cite this article
Zapata, J.M., Krajewska, M., Krajewski, S. et al. Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat 47, 129–140 (1998). https://doi.org/10.1023/A:1005940832123
Issue Date:
DOI: https://doi.org/10.1023/A:1005940832123