Abstract
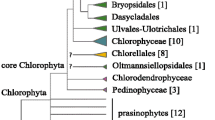
Group I introns were reported for the first time in the large subunit of Rubisco (rbcL) genes, using two colonial green algae, Pleodorina californica and Gonium multicoccum (Volvocales). The rbcL gene of P. californica contained an intron (PlC intron) of 1320 bp harboring an open reading frame (ORF). The G. multicoccum rbcL gene had two ORF-lacking introns of 549 (GM1 intron) and 295 (GM2 intron) base pairs. Based on the conserved nucleotide sequences of the secondary structure, the PlC and GM1 introns were assigned to group IA2 whereas the GM2 intron belonged to group IA1. Southern hybridization analyses of nuclear and chloroplast DNAs indicated that such intron-containing rbcL genes are located in the chloroplast genome. Sequencing RNAs from the two algae revealed that these introns are spliced out during mRNA maturation. In addition, the PlC and GM1 introns were inserted in the same position of the rbcL exons, and phylogenetic analysis of group IA introns indicated a close phylogenetic relationship between the PlC and GM1 introns within the lineage of bacteriophage group IA2 introns. However, P. californica and G. multicoccum occupy distinct clades in the phylogenetic trees of the colonial Volvocales, and the majority of other colonial volvocalean species do not have such introns in the rbcL genes. Therefore, these introns might have been recently inserted in the rbcL genes independently by horizontal transmission by viruses or bacteriophage.
Similar content being viewed by others
References
Bao Y, Herrin DL: Nucleotide sequence and secondary structure of the chloroplast group I intron Cr.psbA2: novel features of this selfsplicing ribozyme. Nucl Acids Res 21: 1667 (1993).
Bechhofer DH, Hue KK, Shub DA: An intron in the thymindylate synthetase gene of Bacillus bacteriophage beta22: evidence for independent evolution of a gene, its group I intron, and the intron open reading frame. Proc Natl Acad Sci USA 91: 11669–11673 (1994).
Burke JM, Belfort M, Cech TR, Davis RW, Schweyen RJ, Shub DA, Szostack JW, Tabak HF: Structural conventions for group I introns. Nucl Acids Res 15: 7217–7221 (1987).
Cech TR: Conserved sequences and structures of group I introns: building an active site for RNA catalysis: review. Gene 73: 259–271 (1988).
Damberger SH, Gutell RR: A comparative database of group I intron structures. Nucl Acids Res 22: 3508–3510 (1994).
De Jonckheere JF, Brown S: Loss of the ORF in the SSUrRNA group I intron of one Naegleria lineage. Nucl Acids Res 22: 3925–3927 (1994).
Gingrich JC, Hallick RB: The Euglena gracilis chloroplast ribulose1,5bisphosphate carboxylase gene. I. Complete DNA sequence and analysis of the nine intervening sequences. J Biol Chem 260: 16156–16161 (1985).
Gingrich JC, Hallick RB: The Euglena gracilis chloroplast ribulose1,5bisphosphate carboxylase gene. II. The spliced mRNAand its product. J Biol Chem 260: 16162–16168 (1985).
Goodrich Blair H, Shub DA: The DNA polymerase genes of several HMVbacteriophages have similar group I introns with highly divergent open reading frames. Nucl Acids Res 22: 3715–3721 (1994).
Harris EH: The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego (1989).
Hedtke B, Borner T, Weihe A: Mitochondrial and chloroplast phagetype RNA polymerases in Arabidopsis. Science 227: 809–811 (1997).
Higgins DG, Bleasby AJ, Fuchs R: CLUSTAL V: improved software for multiple sequence alignment. CABIOS 8: 198–191 (1991).
Johansen S, Johansen T, Faugli F: Structure and evolution of myxomycete nuclear group I introns: a model for horizontal transfer by intron homing. Curr Genet 22: 297–304 (1992).
Kimura M: A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120 (1980).
Kono M, Satoh H, Okabe Y, Abe Y, Nakayama K, Okada M: Nucleotide sequence of the large subunit of ribulose1,5bisphosphate carboxylase/oxygenase from the green alga Bryopsis maxima. Plant Mol Biol 17: 505–508 (1991).
Lambowitz AM, Belfort M: Introns asmobile genetic elements. Annu Rev Biochem 62: 587–622 (1993).
Lipman DJ, Person WR: Rapid and sensitive protein similarity searches. Science 227: 1435–1441 (1985).
Manhart JR, Vonder Haar RA: Intron revealed by nucleotide sequence of large subunit of ribulose1,5bisphosphate carboxylase/oxygenase from Codium fragile (Chlorophyta): phylogenetic analysis. J Phycol 27: 613–617 (1991).
Michel F, Westhof E: Modelling the threedimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol 216: 585–610 (1990).
Nozaki H, Ito M: Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from cladistic analysis based on morphological data. J Phycol 30: 353–365 (1994).
Nozaki H, Ito M, Sano R, Uchida H, Watanabe MM, Kuroiwa T: Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data. J Phycol 31: 970–979 (1995).
Nozaki H, Ito M, Sano R, Uchida H, Watanabe MM, Takahashi H, Kuroiwa T: Phylogenetic analysis of Yamagishiella and Platydorina (Volvocaceae, Chlorophyta) based on rbcL gene sequences. J Phycol 33: 272–278 (1997).
Ohta N, Nagashima H, Kawano S, Kuroiwa, T: Isolation of the chloroplast DNA and the sequence of the trnK gene from Cyanidium caldarium strain RK1. Plant Cell Physiol 33: 657–661 (1992).
Pearson WR: Searching protein sequences libraries: comparison of the sensitivity of the SmithWaterman and FASTA algorithms. Genomics 11: 635–650 (1991).
Pearson WR, Lipman DJ: Imported tools for biological sequence comparison. Proc Natl Acad Sci USA 85: 2444–2448 (1988).
Saitou N, Nei M: The neighborjoining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 (1987).
Siemeister G, Hachtel W: Structure and expression of a gene encoding the large subunit of ribulose1,5bisphosphate carboxylase (rbcL) in the colourless euglenoid flagellate Astasia longa. Plant Mol Biol 14: 825–833 (1991).
Shub DA, Gott JM, Xu MQ, Lang BF, Michel F, Tomaschewski J, Pedersen Lane J, Belfort M: Structural conservation among three homologous introns of bacteriophage T4 and group I introns of eukaryotes. Proc Natl Acad Sci USA 85: 11521–1155 (1988).
Smith TF, Waterman MS: Identification of common molecular subsequences. J Mol Biol 147: 195–197 (1981).
Songsri P, Suzuki S, Fujie M, Yamada T: Horizontal transmission of group I introns mediated by viruses. Nucl Acids Symp Ser 35: 197–198 (1996).
Suzuki K, Ohta N, Kuroiwa T: Isolation of the cellnuclear, mitochondrial, and chloroplast DNA from the ultrasmall eukaryote Cyanidioschyzon merolae. Protoplasma 171: 80–84 (1992).
Thompson JD, Higgins DG, Gibson TJ: CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionspecific gap penalties and weight matrix choice. Nucl Acids Res 22: 4673–4680 (1994).
Turmel M, Boulanger J, Schnare MN, Gray MW, Lemieux C: Six group I introns and three internal transcribed spacers in the chloroplast large subunit ribosomal RNA gene of the green alga Chlamydomonas eugametos. J Mol Biol 218: 293–311 (1991).
Turmel M, Mercier JP, Côté MJ: Group I introns interrupt the chloroplast psaB and psbC and the mitochondrial rrnL gene in Chlamydomonas. Nucl Acids Res 22: 5242–5250 (1993).
Van Oppen MJH, Olsen JL, Stam WT: Evidence for independent acquisition of group I introns in green algae. Mol Biol Evol 10: 1317–1326 (1993).
Watanabe MM, Nozaki H: NIESCollection, List of Strains, Algae and Protozoa, 4th ed. National Institute for Environmental Studies, Tsukuba (1994).
Wilcox LW, Lewis LA, Fuerst PA, Taylor JW: Group I introns within the nuclearencoded smallsubunit rRNA gene of three green algae. Mol Biol Evol 9: 1103–1118 (1992).
Yamada T, Songsri P, Tamura K: Horizontal transmission of groupI ribozymes: viruses as a carrier of the introns. Nucl Acids Symp Ser 34: 119–120 (1995).
Yoshinaga K, Ohta T, Suzuki Y, Sugiura, M: Chlorella chloroplast DNA sequence containing a gene for the subunit of ribulose1,5bisphosphate carboxylase/oxygenase and a part of a possible gene for the beta0 subunit of RNA polymerase. Plant Mol Biol 10: 245–250 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nozaki, H., Ohta, N., Yamada, T. et al. Characterization of rbcL group IA introns from two colonial volvocalean species (Chlorophyceae). Plant Mol Biol 37, 77–85 (1998). https://doi.org/10.1023/A:1005904410345
Issue Date:
DOI: https://doi.org/10.1023/A:1005904410345