Abstract
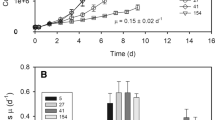
Photoautotrophic suspension cells of Marchantia polymorpha were grown at gas phase CO2 concentrations of 0.4% and 2.0%. At the higher CO2 concentration the chloroplast shape appeared to be modified and the cells had about 70% more chloroplasts per cell. Differences in chlorophyll content per cell were much less pronounced, indicating a reduction in chlorophyll content per chloroplast. Also the cell size was affected by the CO2 concentration, and our data suggest that it was about 37% lower in high CO2 grown cells than in low CO2 grown cells. The capacity and the efficiency of photosynthetic oxygen evolution on a chlorophyll basis and the photosystem II chlorophyll fluorescence parameters were almost identical in both cell types. Immunodection showed that also the ratio of light harvesting complex II antenna proteins and ribulose 1,5 bisphosphate carboxylase/oxygenase were unaltered. These data indicate that the chloroplast density within photoautotrophic culture cells may be regulated independently of their photosynthetic efficiency.
Similar content being viewed by others
References
Buns R, Acker G & Beck E (1993) Plastids of the yew tree (Taxus baccata L.): Ultrastructure and immunocytochemical examination of chloroplastic enzymes. Bot. Acta 106: 32–41
Carrier P, Chagvardieff P & Tapie P (1989) Comparison of the oxygen exchange between photosynthetic cell suspensions and detached leaves of Euphorbia characias L. Plant Physiol. 91: 1075–1079
Chagvardieff P, Péan M, Carrier P & Dimon B (1990) Oxygen exchange capacities in a photoautotrophic batch culture of Euphorbia characias cell suspension. Plant Physiol. Biochem. 28: 231–238
Chow WS & Anderson JM (1987) Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. II. Thylakoid membrane components. Aust. J. Plant Physiol. 14: 9–19
Dracup M, Gibbs J, Stuiver CEE, Greenway H & Flowers TJ (1986) Determination of free space, growth, solute concentrations and parameters of water relations of suspension-cultured tobacco cells. Plant, Cell & Environment 9: 693–701
Genty B, Briantais J-M & Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990: 87–92
Honda SI, Hongladarom-Hond T, Kwanyuen P & Wildman SG (1971) Interpretations on chloroplast reproduction derived from correlations between cells and chloroplasts. Planta 97: 1–15
Hüsemann W & Barz W (1977) Photoautotrophic growth and photosynthesis in cell suspension cultures of Chenopodium rubrum. Physiol. Plant. 40: 77–81
Katoh K (1983) Kinetics of photoautotrophic growth of Marchantia polymorpha cells in suspension culture. Physiol. Plant. 59: 242–248
Kovtun Y & Daie J (1995) End-product control of carbon metabolism in culture-grown sugar beet plants. Plant Physiol. 108: 1647–1656
Krapp A, Hofmann B, Schäfer C & Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the 'sink regulation' of photosynthesis? The Plant J. 2: 817–828
Nagai T, Nakamura C & Nagayoshi T (1989) 2,4-D-sustained photomixotrophic growth of a chlorophyllous cell suspension culture of Nicotiana tabacum. Plant Cell Physiol. 30: 17–23
Ohta Y, Katoh K & Miyake K (1977) Establishment and growth characteristics of a cell suspension culture of Marchantia polymorpha L. with high chlorophyll content. Planta 136: 229–232
Ono K (1973) Callus formation in liverwort, Marchantia polymorpha. Jap. J. Gen. 48: 69–70
Porra RJ, Thompson WA & Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975: 384–394
Possingham JV& Lawrence ME(1983) Controls of plastid division. Int. Rev. Cytol. 84: 1–56
Possingham JV & Smith JW (1972) Factors affecting chloroplast replication in spinach. J. Exp. Bot. 23: 1050–1059
Pyke KA & Leech RM (1987) The control of chloroplast number in wheat mesophyll cells. Planta 170: 416–420
Pyke KA & Leech RM (1992) Chloroplast division and expansion is radically altered by nuclear mutations in Arapidopsis thaliana. Plant Physiol. 99: 1005–1008
Pyke KA & Leech RM (1994) A genetic analysis of chloroplast division and expansion in Arapidopsis thaliana. Plant Physiol. 104: 201–207
Reski R, Wehe M, Kasten B, Reutter K, Marienfeld JR & Abel WO (1992) The molecular analysis of chloroplast division. Crypt. Bot. 3: 18–22
Reynolds DS (1963) The use of lead citrate at high pH as an opaque stain in electron microscopy. J. Cell Biol. 17: 208–212
Schäfer C & Schmid V (1995) The use of photoautotrophic plant cells as a model system in studies on light stress and photoinhibition. In: Carré F & Chagvardieff P (eds) Ecophysiology and Photosynthetic in vitro Cultures (pp 47–54). CEA, Cadarache
Schäfer C & Schmidt E (1991) Light acclimation potential and xanthophyll cycle pigments in photoautotrophic suspension cells of Chenopodium rubrum. Physiol. Plant. 82: 440–448
Schäfer C, Simper H & Hofmann B (1992) Glucose feeding results in coordinated changes of chlorophyll content, ribulose-1,5-bisphosphate carboxylase-oxygenase activity and photosynthetic potential in photoautotrophic suspension cultured cells of Chenopodium rubrum. Plant, Cell and Environm. 15: 343–350
Schäfer C, Vogg G & Schmid V (1993) Evidence for loss of D1 protein during photoinhibition of ChenopodiumrubrumL. culture cells. Planta 189: 433–439
Schmid V& Schäfer C (1994) Alterations of the chlorophyll-protein pattern in chronically photoinhibited Chenopodium rubrum cells. Planta 192: 473–479
Schmid V (1995) Lichtstreßbedingte Änderungen in der Menge, der Verteilung und im Umsatz photosynthetischer Pigment-Protein-Komplexe bei photoautotrophen Chenopodium rubrum Suspensionskulturzellen. Verlag Shaker, Aachen
Schreiber U, Bilger W & Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D & Caldwell MM (eds) Ecophysiology of Photosynthesis, Ecological studies, Vol 100 (pp 49–70). Springer, Berlin
Spurr AR (1969) A low-viscosy epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26: 31–43
Takeda S, Sato F & Yamada Y (1989) Photosynthetic characteristics of photoautotrophically cultured cells of tobacco. Plant Cell Physiol. 30: 885–891.
Terashima I & Evans JR (1988) Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 19: 143–155
Tichá I, Schäfer C & Capková C (1995) Photoinhibition in photoautotrophic tobacco plantlets in vitro. In: Mathis P (ed) Photosynthesis: from Light to Biosphere, Vol IV (pp 445–448). Kluwer, Dordrecht
Van Oosten J-J, Wilkins D & Besford RT (1994) Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by regulation by carbohydrates: a mechanism for the acclimation of photosynthesis to high CO2? Plant, Cell and Environm. 17: 913–923
Widholm J (1992) Properties and uses of photoautotrophic plant cell cultures. Int. Rev. Cytol. 132: 109–175
Ziegler P & Beck E (1986) Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 82: 1119–1121
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bockers, M., Capková, V., Tichá, I. et al. Growth at high CO2 affects the chloroplast number but not the photosynthetic efficiency of photoautotrophicMarchantia polymorpha culture cells. Plant Cell, Tissue and Organ Culture 48, 103–110 (1997). https://doi.org/10.1023/A:1005893311140
Issue Date:
DOI: https://doi.org/10.1023/A:1005893311140