Abstract
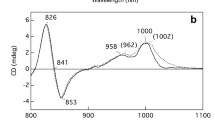
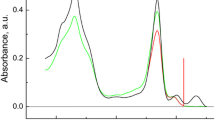
Fluorescence detected magnetic resonance (FDMR) was used to study the lowest triplet state of bacteriochlorophylls (BChls) c and d in Chlorobium (Chl.) tepidum and Chl. vibrioforme, respectively. These pigments were studied both in the oligomeric form (in whole cells) and in the monomeric form (after conversion using a 1% 1-hexanol treatment). Fluorescence spectra show the presence of lower-state aggregates, apart from monomers, in samples treated with 1-hexanol. Values of the zero field splitting (ZFS) parameter D, obtained from FDMR spectra, were found to decrease with an increasing aggregate size. The observed ZFS trends are explained by a delocalization of the triplet spins, including a charge resonance (CR) contribution, over the aggregate. A simple model is presented relating the changes of D and E as a result of monomer aggregation to the aggregate geometry. Application of this model to BChls c and d indicates approximately diagonal stacking of the monomers in the dimer. Results for oligomeric BChl c and d were compared with those previously obtained for oligomeric BChl e. FDMR transitions of BChls c, d and e differ both in frequencies and in signs. The D and E values of Car's and BChl a (in whole cells) agree well with those reported for Chl. phaeobacteroides and Chl. limicola.
Similar content being viewed by others
References
Bixon M, Fajer J, Feher G, Freed JH, Gamliel D, Hoff AJ, Levanon H, Möbius K, Nechushtai R, Norris JR, Cherz A, Sessler JL and Stehlik D (1992) Primary events in photosynthesis: Problems, speculations, controversies, and future trends. Isr J Chem 32: 364-511 1992
Boender G-J, Balaban TS, Holzwarth AR, Schaffner K, Raap J, Prytulla S, Oschkinat H and De Groot HJM (1995) Comparison of the stacking of chlorophylls in chlorosomes versus aggregates of bacterioclorophyll c and chlorophyll a using 2-D MAS NMR spectroscopy. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, Vol I, pp 347-350, Kluwer Academic Publishers, Dordrecht
Brune DC, Nozawa T and Blankenship RE (1987) Antenna organization in green photosynthetic bacteria. 1. Oligomeric bacteriochlorophyll c as a model for the 740 nm absorbing bacteriochlorophyll c in Chloroflexus aurantiacus chlorosomes. Biochemistry 26: 8644-8652
Brune DC, King GH and Blankenship RE (1988) Interactions between Bacterichlorophyll c molecules in oligomers and in chlorosomes of green photosynthetic bacteria. In: Scheer H and Schneider S (eds) Photosynthetic Light-Harvesting Systems, pp 141-151. De Gruyter, Berlin, New York
Buck DR and Struve WS (1996) Tubular exciton models for BChl c antennae in chlorosomes from green photosynthetic bacteria. Photosynth Res 48: 367-377
Causgrove TP, Cheng P, Brune DC and Blankenship RE (1993) Optical spectroscopy of highly fluorescent aggregate of bacteriochlorophyll c. J Phys Chem 97: 5519-5524
Fetisova ZG, Kharchenko and Abdourakhamanov IA (1986) Strong orientational ordering of the near-infrared transition moment vectorts of light-harvesting antenna bacterioviridin in chromatophores of the green photosynthetic bacterium Chlorobium limicola. FEBS Lett 199: 234-236
Griebenow K and Holzwarth AR (1989) Pigment organization and energy transfer in green bacteria. 1. Isolation of native chlorosomes free of bound bacteriochlorophyll a from Chloroflexus aurantiacus by gel-electrophoretic filtration. Biochim Biophys Acta 973: 235-240
Hoff AJ (1986) Optically detected magnetic resonance (ODMR) of triplet states in vivo. In: Staehelin LA and Arntzen CJ (eds) Photosynthesis III (Encyclopedia of Plant Physiology), pp 400-421. Springer-Verlag, Berlin
Hojrup P, Gerola P, Hansen HF, Mikkelsen JM, Shahed AE, Knudsen, Roepstorf P and Olson JM (1991) The amino acid sequence of a major protein component in the light harvesting complex of the green photosynthetic bacterium Chlorobium limicola f. thiosulfatum. Biochim Biophys Acta 1077: 220
Holzwarth AR and Schaffner K (1994) On the structure of bacteriochlorophyll molecularaggregates in the chlorosomes of green bacteria. A molecular modelling study. Photosynth Res 41: 25-233
Kooyman RPH, Schaafsma TJ (1980) A charge resonance-exciton model of molecular dimers. J Mol Struct 60: 373-380
Lemaistre JP and Zewail AH (1979) Fluorescence, phosphorescence and ODMR line narrowingof molecules in solids. Chem Phys Lett 68: 302-308
Matsuura K and Olson JM (1990) Reversible conversion of aggregated bacteriochlorophyll c to the monomeric form by 1-hexanol in chlorosomes from Chlorobium and Chloroflexus. Biochim Biophys Acta 1019: 233-238
Matsuura K, Hirota M, Shimada M and Mimuro M (1993) Spectral forms and orientation of bacteriochlorophylls c and a in chlorosomes of green photosynthetic bacterium Chloroflexus aurantiacus. Photochem Photobiol 57: 92-97
Miller M, Gilbro T and Olson JM (1993) Aqueous aggregates of bacteriochlorophyll c as a model for pigment organization in chlorosomes. Photochem Photobiol 57: 98-102
Norris JR, Sheer H and Katz JJ (1975) Models for antenna and reaction center chlorophylls. Ann NY Acad Sci USA 244: 260-280
Nozawa T, Ohmoto K, Suzuki M, Nakagawa H, Shikama Y, Konami H and Wang Z-Y (1994) Strucutures of chlorosomes and aggregated BChl c in Chlorobium tepidum from solid state high resolution CP/MAS 13C NMR. Photosynth Res 41: 211-223
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594: 33-51, 1980
Olson JM and Pedersen JP (1988) Bacteriochlorophyll c aggregates in carbon tetrachloride asmodels for chlorophyll organization in green photosynthetic bacteria. In: Scheer H and Schneider S (eds) Photosynthetic Light-Harvesting Systems, pp 365-373. De Gruyter, Berlin, New York
Psencik J, Searle GFW, Hála J and Schaafsma TJ (1994) Fluorescence detected magnetic resonance (FDMR) of green sulfur photosynthetic bacteria Chlorobium sp. Photosynth Res 40: 1-10
Schaafsma TJ (1982) ODMR in Photosynthesis I. In: Clarke RH (ed) Triplet state ODMR Spectroscopy, pp 367-425. Wiley-Interscience, New York
Schweitzer D, Hausser KH, Taglieber V and Staab HA (1976) Electronic properties of two isomeric charge-transfer [2.2] paracyclophanes. Chem Phys 14: 183-187
Smith KM, Kehres LA and Fajer J (1983) Aggregation of the bacteriochlorophylls c, d and e. Models for the antenna chlorophylls of green and brown photosynthetic bacteria. J Am Chem Soc 105: 1387-1389
Smith KM, Bobe FW, Goff DA and Abraham RJ (1986) NMR spectroscopy of porphyrins, 28. Detailed solution structure of a bacteriochlorophyllide d dimer. J Am Chem Soc 108: 1111-1120
Sternlicht H and McConnel HM (1961) Paramagnetic excitons in molecular crystals. J Chem Phys 35: 1793
Uehara K and Olson JM (1992) Aggregation of bacteriochlorophyll c homologs to dimers, tetramers and polymers in water-saturated carbon tetrachloride. Photosynth Res 33: 251-257
Van Dorssen RJ, Gerola PD, Olson JM and Amesz J (1986) Optical and structural properties of chlorosomes of the photosynthetic green sulfur bacterium Chlorobium limicola. Biochim Biophys Acta 848: 77-82
Wasielewski R (1993) Modelling primary electron transfer in photosynthesis using supramolecular structures. In: Deisenhofer J and Norris JR (eds) The Photosynthetic Reaction Center, Vol II. Academic Press, San Diego, CA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Psencik, J., Schaafsma, T.J., Searle, G.F.W. et al. Fluorescence detected magnetic resonance of monomers and aggregates of bacteriochlorophylls of green sulfur bacteria Chlorobium sp.. Photosynthesis Research 52, 83–92 (1997). https://doi.org/10.1023/A:1005842231286
Issue Date:
DOI: https://doi.org/10.1023/A:1005842231286