Abstract
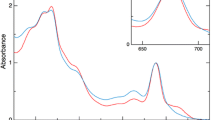
Low-temperature (77 K) fluorescence emission spectra of intact cells of a cyanobacterium, Synechocystis sp. PCC 6714, and a green alga, Chlamydomonas reinhardtii, were quantitatively analyzed to examine differences in PS I/PS II stoichiometries. Cells cultured under different spectral conditions had various PS I/PS II molar ratios when estimated by oxidation-reduction difference absorption spectra of P700 (for PS I) and Cyt b-559 (for PS II) with thylakoid membranes. The fluorescence emission spectra under the Chl a excitation at 435 nm were resolved into several component bands using curve-fitting methods and the relative band area between PS II (F685 and F695) and PS I (F710 or F720) emissions was compared with the PS I/PS II stoichiometries of the various cell types. The results indicated that the PS I/PS II fluorescence ratios correlated closely with photosystem stoichiometries both in Synechocystis sp. PCC 6714 and in C. reinhardtii grown under different light regimes. Furthermore, the correlation between the PS I/PS II fluorescence ratios and the photosystem stoichiometries is also applicable to vascular plants.
Similar content being viewed by others
References
Aizawa K and Fujita Y (1997) Regulation of synthesis of PS I in the cyanophytes SynechocystisPCC 6714 and Plectonema boryanumduring the acclimation of the photosystem stoichiometry to the light quality. Plant Cell Physiol 38: 319-326
Aizawa K, Shimizu T, Hiyama Y, Satoh K, Nakamura Y and Fujita Y (1992) Changes in composition of membrane proteins accompanying the regulation of PS I/PS II stoichiometry observed with SynechocystisPCC 6803. Photosynth Res 32: 131-138
Ajlani G, Vernotte C, Dimagno L and Haselkorn R (1995) Phycobilisome core mutants of SynechocystisPCC 6803. Biochim Biophys Acta 1231: 189-196
Allen JF, Mullineaux CW, Sanders CE and Melis A (1989) State transitions, photosystem stoichiometry adjustment and non-photochemical quenching in cyanobacterial cells acclimated to light absorbed by Photosystem I or Photosystem II. Photosynth Res 22: 157-166
Bruce D, Brimble S and Bryant DA (1989) State transitions in a phycobilisome-less mutant of the cyanobacterium Synechococcussp. PCC 7002. Biochim Biophys Acta 974: 66-73
Butko P (1984) Changes in photosynthetic apparatus and excitation energy distribution in Chlorelladuring the life cycle. Photobiochem Photobiopys 8:63-72
Butler WL and Kitajima M (1975) Energy transfer between Photosystem II and Photosystem I in chloroplasts Biochim Biophys Acta 396: 72-85
Chow WS, Anderson JA, and Hope AB (1988) Variable stoichiometries of Photosystem II to Photosystem I reaction centers. Photosynth Res 17: 277-281
Cunningham FX Jr, Dennenberg RJ, Mustardy L, Jursinic PA and Gantt E (1989) Stoichiometry of Photosystem I, Photosystem II, and phycobilisomes in the red alga Porphyridium cruentumas a function of growth irradiance. Plant Physiol. 91: 1179-1187
Cunningham FX Jr, Dennenberg RJ, Jursinic PA and Gantt E (1990) Growth under red light enhances Photosystem II relative to Photosystem I and phycobilisomes in the red alga Porphyridium cruentum. Plant Physiol 93: 888-895
Delphin E, Duval J-C and Kirilovsky D (1995) Comparison of state 1-state 2 transitions in the green alga Chlamydomonas reinhardtiiand in the red alga Rhodella violacea: Effect of kinase and phosphatase inhibitors. Biochim Biophys Acta 1232: 91-95
Fork DC and Satoh K (1986) The control by state transitions of the distribution of excitation energy in photosynthesis. Ann Rev Plant Physiol 37: 335-361
Fujita Y and Murakami A (1987) Regulation of electron transport composition in cyanobacterial photosynthetic system: Stoichiometry among Photosystem I and II complexes and their light-harvesting antennae and cytochrome b 6-fcomplex. Plant Cell Physiol 28: 1547-155
Fujita Y, Ohki K and Murakami A (1985) Chromatic regulation of photosystem composition in the photosynthetic system of red and blue-green algae. Plant Cell Physiol 26: 1541-1548
Fujita Y, Murakami A, Ohki K and Hagiwara N (1988) Regulation of photosystem composition in cyanobacterial photosynthetic system: Evidence indicating that Photosystem I formation is controlled in response to the ellectron transport state. Plant Cell Physiol 29: 557-564
Fujita Y, Murakami A, Aizawa K and Ohki K (1994) A short-term and long-term adaptation of the photosynthetic apparatus: Homeostatic properties of thylakoids. In: Bryant DA (ed) The Molecular Biology of Cyanobacteria, pp 677-692. Kluwer Academic Publishers, Dordrecht, the Netherlands
Fujita Y, Murakami A and Aizawa K (1995) The accumulation of protochlorophyllide in cells of SynechocystisPCC 6714 with a low PS I/PS II stoichiometry. Plant Cell Physiol 36: 575-582
Garewall HS and Wasserman AR (1974) Triton X-100-4M urea as an extraction medium for membrane proteins. I. Purification of chloroplast cytochrome b-559. Biochemistry 13: 4063-4071
Glick RE, McCauley SW, Gruissem W and Melis A (1986) Light quality regulates expression of chloroplast genes and assembly of photosynthetic membrane complexes. Proc Natl Acad Sci USA 83: 4287-4291
Gu T-Q, Iwama Y, Murakami A, Adhikary SP and Fujita Y (1994) Changes in the cytochrome coxidase activity in response to light regime for photosynthesis observed with the cyanophyte SynechocystisPCC 6714. Plant Cell Physiol 35: 1135-1140
Hiyama T and Ke B (1972) Difference spectra and extinction coefficients of P700. Biochim Biophys Acta 267: 160-171
Jursinic P and Dennenberg R (1989) Measurement of stoichiometry of Photosystem II to Photosystem I reaction centers. Photosynth Res 21: 197-200
Kawamura M, Mimuro M and Fujita Y (1979) Quantitative relationship between two reaction centers in the photosynthetic system of blue-green algae. Plant Cell Physiol 20: 697-705
Kim JH, Glick RE and Melis A (1993) Dynamics of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol 102: 181-190
Kuwabara T and Murata N (1982) Inactivation of photosynthetic oxygen evolution and concomitant release of three polypeptides in the Photosystem II particles of spinach chloroplasts. Plant Cell Physiol 23: 533-539
Ley CA and Butler WL (1980) Effects of chromatic adaptation on the photochemical apparatus of photosynthesis in Porphyridium cruentum. Plant Physiol. 65: 714-722
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315-322
MacDonald GM, Boerner RJ, Everly RM, Cramer WA, Debus RJ and Barry BA (1994) Comparison of cytochrome b-559 content in Photosystem II complexes from spinach and Synechocystisspecies PCC 6803. Biochemistry 34: 4393-4400
Manodori A and Melis A (1984) Photochemical apparatus organization in Anacystis nidulans(Cyanophyceae): Effect of CO2concentration during cell growth. Plant Physiol 74: 67-71
Melis A, Mullineaux CW and Allen JF (1989) Acclimation of the photosynthetic apparatus to Photosystem I or Photosystem II light: Evidence from quantum yield measurements and fluorescence spectroscopy of cyanobacterial cells. Z Naturforsch 44C: 109-118
Melis A, Murakami A, Nemson JF, Aizawa K, Ohki K and Fujita Y (1996) Chromatic regulation in Chlamydomonas reinhardtiialters photosystem stoichiometry and improves the quantum efficiency of photosynthesis. Photosynth Res 47: 253-265
Mende D, Maroti P and Wiessner W (1983) Energy distribution between two photosystems during the life-cycle of synchronized cultures of Chlorella fusca. Physiol Veg 21: 469-474
Mimuro M and Fujita Y (1977) Estimation of chlorophyll adistribution in the photosynthetic pigment system I and II of the blue-green alga Anabaena variabilis. Biochim Biophys Acta 459: 376-389
Mimuro M, Murakami A and Fujita Y (1982) Studies on spectral characteristics of allophycocyanin isolated from Anabaena cylindrica: Curve-fitting analysis. Arch Biochem Biophys 215: 266-273
Mimuro M, Tamai N, Yamazaki T and Yamazaki I (1987) Excitation energy transfer in spinach chloroplasts: Analysis by the time-resolved fluorescence spectrum at −196 °C in the picosecond time range. FEBS Lett 213: 119-122
Murakami A and Fujita Y (1988) Steady state of photosynthesis in cyanobacterial photosynthetic system before and after regulation of electron transport composition: Overall rate of photosynthesis and PS I/PS II composition. Plant Cell Physiol 29: 305-311
Murakami A and Fujita Y (1991a) Steady state of photosynthetic electron transport in cells of the cyanophyte SynechocystisPCC 6714 having different stoichiometry between PS I and PS II: Analysis of flash-induced oxidation-reduction of cytochrome fand P700 under steady state of photosynthesis. Plant Cell Physiol 32: 213-222
Murakami A and Fujita Y (1991b) Regulation of photosystem stoichiometry in the photosynthetic system of the cyanophyte SynechocystisPCC 6714 in response to light-intensity. Plant Cell Physiol 32: 223-230
Murakami A, Fujita Y, Nemson JF and Melis A (1997) Chromatic regulation in Chlamydomonas reinhardtii: Time course of photosystem stoichiometry adjustment following a shift in growth light quality. Plant Cell Physiol 38: 188-193
Murata N (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll afluorescence in Porphyridium cruentum. Biochim Biophys Acta 172: 242-251
Murata N, Nishimura M and Takamiya A (1966) Fluorescence of chlorophyll in photosynthetic systems. III. Emission and action spectra of fluorescence — three emission bands of chlorophyll aand the energy transfer between two pigment systems. Biochim Biophys Acta 126: 234-243.
Myers J, Graham JR and Wang RT (1980) Light harvesting in Anacystis nidulansstudied in pigment mutants. Plant Physiol 66: 1144-1149
Nilsson F, Simpson DJ, Jansson C and Andersson B (1992) Ultrastructural and biochemical characterization of a Synechocystis6803 mutant with inactivated psbAgenes. Arch Biochim Biophys 295: 340-347
Salehian O and Bruce D (1992) Distribution of excitation energy in photosynthesis: Quantification of fluorescence yields from intact cyanobacteria. J Luminescence 51: 91-98
Satoh K (1980) F-695 emission from the purified Photosystem II chlorophyll a-protein complex. FEBS Lett 110: 53-56
Schubert H and Hagemann M (1990) Salt effects on 77 K fluorescence and photosynthesis in the cyanobacterium Synechocystissp. PCC 6803. FEMS Microbiol Lett 71: 169-172
Shen G and Vermaas WFJ (1994) Chlorophyll in a Synechocystissp. PCC 6803 mutant without Photosystem I and Photosystem II core complexes. Evidence for peripheral antenna chlorophylls in cyanobacteria. J Biol Chem 269: 13904-13910
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 46: 83-91
Vernotte C, Astier C and Olive J (1990) State 1-state 2 adaptation in the cyanobacteria SynechocystisPCC 6714 wild type and SynechocystisPCC 6803 wild and phycocyanin-less mutant. Photosynth Res 26: 203-212
Watanabe A (1960) List of algal strains in collection at the Institute of Applied Microbiology, University of Tokyo. J Gen Appl Microbiol 6: 28-292
Westermann M, Ernst A, Brass S, Böger P and Wehrmeyer W (1994) Ultrastructure of cell wall and photosynthetic apparatus of the phycobilisome-less Synechocystissp. Strain BO 8402 and phycobilisome-containing derivative strain BO 9201. Arch Microbiol 162: 222-232
Wilhelm C (1990) The biochemistry and physiology of light-harvesting processes in chlorophyll b-and chlorophyll c-containing algae. Plant Physiol Biochem 28: 293-306.
Williams WP and Allen JF (1987) State 1/state 2 changes in higher plants and algae. Photosynth Res 13: 19-45
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murakami, A. Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PS I/PS II stoichiometries. Photosynthesis Research 53, 141–148 (1997). https://doi.org/10.1023/A:1005818317797
Issue Date:
DOI: https://doi.org/10.1023/A:1005818317797