Abstract
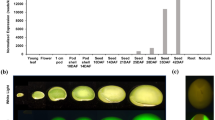
We have designed protein molecules based on an α-helical coiled-coil structure. These proteins can be tailored to complement nutritionally unbalanced seed meals. In particular, these proteins may contain up to 43% mol/mol of the essential amino acid lysine. Genes encoding such proteins were constructed using synthetic oligonucleotides and the protein stability was tested for in vivo by expression in an Escherichia coli model system. A protein containing 31% lysine and 20% methionine (CP 3-5) was expressed in transgenic tobacco seeds utilizing the seed specific bean phaseolin and soybean β-conglycinin promoters. Both promoters provided a level of expression in the mature transgenic tobacco seeds which resulted in a significant increase in the total lysine content of the seeds. Several of these transgenic lines were analyzed for three generations to determine the stability of gene expression. Plants transformed with the soybean β-conglycinin promoter/CP 3-5 gene consistently expressed the high-lysine phenotype through three generations. However, expression of the high-lysine phenotype in plants transformed with the bean phaseolin/CP 3-5 was variable. This is the first report of a significant increase in seed lysine content due to the seed-specific expression of a de novo protein sequence.
Similar content being viewed by others
References
Altenbach SB, Kuo C, Staraci LC, Pearson KW, Wainwright C, Georgescu A, Townsend J: Accumulation of a brazil nut protein in seeds of transgenic canola results in enhanced levels of seed protein methionine. Plant Mol Biol 18: 235-245 (1992).
Altenbach SB, Pearson KW, Leung FW, Sun SSM: Cloning and sequence analysis of a cDNA encoding a Brazil nut protein exceptionally rich in methionine. Plant Mol Biol 8: 239-250 (1987).
Altenbach SB, Pearson KW, Meeker G, Staraci LC, Sun SSM: Enhancement of the methionine content of seed proteins by the expression of a chimeric gene encoding a methionine-rich protein in transgenic plants. Plant Mol Biol 13: 513-522 (1989).
Beachy RN, Chen Z-L, Horsch RB, Rogers SG, Hoffman NJ, Fraley RT: Accumulation and assembly of soybean betaconglycinin in seeds of transformed petunia plants. EMBO J 4: 3047-3053 (1985).
Beauregard M, Dupont C, Teather RM, Hefford MA: Design, expression, and initial characterization of MBI, a De Novo protein enriched in essential amino acids. Bio/technology 13: 974-981 (1995).
Brandle JE, McHugh SG, James L, Labbe H, Miki BL: Instability of transgene expression in field grown tobacco carrying the csr1-1 gene for sulfonylurea herbicide resistance. Bio/technology 13: 994-998 (1995).
Campbell WH, Gowri G: Codon usage in higher plants, green algae, and Cyanobacteria. Plant Physiol 92: 1-11 (1990).
Carpino LA, Han GY: The 9 fluorenylmethoxycarbonyl aminoprotecting group. J Org Chem 37: 3404 (1972).
Chou PY, Fasman GD: Prediction of the secondary structure of proteins from amino acid sequence. Adv Enzymol 47: 45-148 (1978).
Cohen C, Parry DAD: Alpha-helical coiled coils: a widespread motif in proteins. Trends Biochem Sci 11: 245-248 (1986).
Cohen C, Parry DAD: Alpha-helical coiled coils: more facts and better predictions. Science 263: 488-489 (1994).
Coleman GD, Chen THH, Fuchigami LH: Complementary DNA cloning of poplar bark storage protein and control of its expression by photoperiod. Plant Physiol 98: 687-693 (1992).
DeClercq A, Vandewiele M, Van Damme J, Guerche P, Van Montagu M, Vandekerckhove J, Krebbers E: Stable accumulation of modified 2S albumin seed storage proteins with higher methionine content in transgenic plants. Plant Physiol 94: 970- 979 (1990).
DeGrado WF, Wasserman ZR, Lear JD: Protein design, a minimalist approach. Science 243: 622-628 (1989).
Ellis JR, Shirsat AH, Hepher A, Yarwood JN, Gatehouse JA, Croy RRD, Boulter D: Tissue-specific expression of a pea legumin gene in seeds of Nicotiana plumbaginifolia. PlantMol Biol 10: 203-214 (1988).
van den Elzen PJM, Townsend J, Lee KY, Bedbrook JR: A chimaeric hygromycin resistance gene as a selectable marker in plant cells. Plant Mol Biol 5: 299-302 (1985).
Harpster MH, Townsend JA, Jones JDG, Bedbrook J, Dunsmuir P: Relative strengths of the 35S califlower mosaic virus, 1′, 2′, and nopaline synthase promoters in transformed tobacco sugarbeet and oilseed rape callus tissue. Mol Gen Genet 212: 182-190 (1988).
Higgins TJV, Chandler PM, Randall PJ, Spencer D, Beach LR, Blagrove RJ, Kortt AA, Inglis AS: Gene structure, protein structure, and regulation of the synthesis of a sulfur-rich protein in pea seeds. J Biol Chem 261: 11 124-11 130 (1986).
Hirai SI, Yaniv M: Jun DNA-binding is modulated by mutations between the leucines or by direct interaction of Fos with the TGACTCA sequence. New Biol 1: 181-191 (1989).
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA: A binary plant vector strategy based on separation of vir-and Tregion of the Agrobacterium tumefaciensTi-plasmid. Nature 303: 179-180 (1983).
Hoffman LM, Donaldson DD, Herman EM: Amodified storage protein is synthesized, processed, and degraded in the seeds of transgenic plants. Plant Mol Biol 11: 717-729 (1988).
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Biol Sci 1229-1231 (1985).
Hu JC, O'shea EK, Kim PS, Sauer RT: Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science 250: 1400-1403 (1990).
Itoh Y, Watson JM, Haas D, Leisinger T: Genetic andmolecular characterization of the Pseudomonasplasmid pVS1. Plasmid 11: 206-220 (1984).
Jaynes JM: De novodesigned synthetic plant storage proteins: enhancing protein quality of plants for improved human and animal nutrition. Biotechnol Feed Ind Proc Alltech's Annu Symp 10: 129-153 (1994).
Jaynes JM, Yang MS, Espinoza N, Dodds JH: Plant protein improvement by genetic engineering: use of synthetic genes. Trends Biotechnol 12: 314-320 (1986).
Katz ED, Dong MW: Rapid analysis and purification of polymerase chain reaction products by high-performance liquid chromatography. Biotechniques 8: 546-554 (1990).
Keeler SJ, Sanders P, Smith JK, Mazur BJ: Regulation of tobacco acetolactate synthase gene expression. Plant Physiol 102: 1009-1018 (1993).
Kirihara JA, Hunsperger JP, Mahoney WC, Messing JW: Differential expression of a gene for a methionine-rich storage protein in maize. Mol Gen Genet 211: 477-484 (1988).
Kirihara JA, Petri JB, Messing J: Isolation and sequence of a gene encoding a methionine-rich 10-kDa zein protein from maize. Gene 71: 359-370 (1988).
Kouzarides T, Ziff E: Behind the Fos and Jun leucine zipper. Cancer Cells 1: 71-76 (1989).
Krebbers E, Rompaey JV, Vandekerckhove J: Expression of modified seed storage proteins in transgenic plants. In: Hiatt A (Ed) Transgenic Plants, pp. 37-60. Marcel Dekker, New York (1993).
Landschulz WH, Johnson PF, McKnight SL: The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240: 1759-1764 (1988).
Lau SYM, Taneja AK, Hodges RS: Synthesis of a model protein of defined secondary and quarternary structure. J Biol Chem 259: 13 253-13 261 (1984).
Liu BL, Jing YX, Kuang TY: Screening of lysine rich plant species and identification of the purified proteins. Acta Bot Sin 35: 62-68 (1993).
Merrifield RB: Peptide synthesis on a solid polymer. Fed Proc Am Soc Exp Biol 21: 412 (1962).
Murashige T, Skoog F: A revised medium for rapid growth bioassays with tobacco tissue cultures. Physiol Plant 15: 473- 497 (1962).
Naito S, Dube PH, Beachy RN: Differential expression of conglycinin alpha prime and beta subunit genes in transgenic plants. Plant Mol Biol 11: 109-123 (1988).
O'Neil KT, DeGrado WF: A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 250: 646-651 (1990).
Pederson K, Argos P, Naravana SVL, Larkins BA: Sequence analysis and characterization of a maize gene encoding a highsulfur zein protein ofMW 15 000. J Biol Chem 261: 6279-6284 (1986).
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA: Modification of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci USA 88: 3324-3328 (1991).
Rafalski M, Ortiz A, Rockwell A, Van Ginkel LC, Lear JD, Degrado WF, Wilschut J: Membrane fusion activity of the influenza virus hemagglutinin: interaction of HA2 N-terminal peptides with phospholipid vesicles. Biochemistry 30: 10 211- 10 220 (1991).
Ransone LJ, Visvader J, Lamph WW, Sassone-Corsi P, Verma IM: Fos and Jun interaction: the role of the leucine zipper. Int J Cancer 4: 10-21 (1989).
Rosenberg AH, Lade BN, Chui D, Lin SW, Dunn JJ, Studier FW: Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene 56: 125-135 (1987).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Schmidt RJ, Burr FA, Aukerman MJ, Burr B: Maize regulatory gene opaque-2 encodes a protein with a ‘leucine-zipper’ motif that binds to zein DNA. Proc Natl Acad Sci USA 87: 46-50 (1990).
Sengupta-Gopalan C, Reichert NA, Barker RF, Hall TC, Kemp JD: Developmentally regulated expression of the bean betaphaseolin gene in tobacco seed. Proc Natl Acad Sci USA 82: 3320-3324 (1985).
Slightom JL, Drong RF, Sieu LC, Chee PC: Custom polymerase chain reaction engineering plant expression vectors and genes for plant expression. In: Gelvin SB, Schilperoort RA (eds) Plant Molecular Biology Manual B16: 1-55. Kluwer Academic Publishers, Dordrecht, Netherlands (1991).
Smith DB, Corcoran LM: Expression and purification of glutathione-S-transferase fusion proteins. In: Current Protocols in Molecular Biology, pp. 16.7.1-16.7.8. JohnWiley, New York (1989).
Studier FW, Moffatt BA: Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189: 113-130 (1986).
Sueki M, Lee S, Power SP, Denton JB, Konishi Y, Scheraga H: Helix-coil stability constants for the naturally occurring amino acids in water. Macromolecules 17: 148-155 (1984).
Townsend JA, Thomas LA: Factors which influence the Agrobacterium-mediated transformation of soybean. J Cell Biochem (Suppl) 18: 78 (1994).
Ueng P, Galili G, Sapanara V, Goldsbrough PB, Dube P, Beachy RN, Larkins BA: Expression of a maize storage protein gene in petunia plants is not restricted to seeds. Plant Physiol 86: 1281-1285 (1988).
Wendoloski JJ, Salemme FR: Probit-A statistical approach to modeling proteins from partial coordinate data using substructure libraries. J Mol Graph 10: 124-126 (1992).
Yang MS, Espinoza NO, Nagpala PG, Dodds JH, White FF, Schnorr KL, Jaynes JM: Expression of a synthetic gene for improved protein quality in transformed potato plants. Plant Sci 64: 99-111 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Keeler, S.J., Maloney, C.L., Webber, P.Y. et al. Expression of de novo high-lysine α-helical coiled-coil proteins may significantly increase the accumulated levels of lysine in mature seeds of transgenic tobacco plants. Plant Mol Biol 34, 15–29 (1997). https://doi.org/10.1023/A:1005809900758
Issue Date:
DOI: https://doi.org/10.1023/A:1005809900758