Abstract
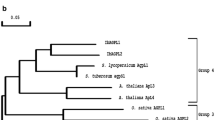
Two cDNA clones (SAS1 and SAS2) encoding different isoforms of asparagine synthetase (AS; EC 6.3.5.4) were isolated. Their DNA sequences were determined and compared. The amino-terminal residues of the predicted SAS1 and SAS2 proteins were identical to those of the glutamine binding domain of AS from pea, asparagus, Arabidopsis and human, suggesting that SAS1 and SAS2 cDNAs encode the glutamine-dependent form of AS. The open reading frames of SAS1 and SAS2 encode a protein of 579 and 581 amino acids with predicted molecular weights of 65 182 and 65 608 Da respectively. Similarity of the deduced amino acid sequences of SAS1 and SAS2 with other known AS sequences were 92% and 93% for pea AS1; 91% and 96% for pea AS2; 88% and 91% for asparagus; 88% and 90.5% for Arabidopsis; 70.5% and 72.5% for E. coli asnB and 61% and 63% for man. A plasmid, pSAS2E, was constructed to express the soybean AS protein in Escherichia coli. Complementation experiments revealed that the soybean AS protein was functional in E. coli. Southern blot analysis indicated that the soybean AS is part of a small gene family. AS transcript was expressed in all tissues examined, but higher levels were seen in stem and root of light-grown tissue and leaves of dark-treated tissue.
Similar content being viewed by others
References
Andrulis IL, Chen J, Ray PN: Isolation of human cDNAs for asparagine synthetase and expression in Jensen rat sarcoma cells. Mol Cell Biol 7: 2435–2443 (1987).
Bachmann BJ, Low KB: Linkage map of Escherichia coli K-12. Microbiol Rev 44: 1–56 (1980).
Bewsey KE, Johnson ME, Huff JP: Rapid isolation and puri-fication of DNA from agarose gels: the phenol-freeze fracture method. Biotechniques 6: 724–725 (1991).
Boehlein SK, Richards NGJ, Walworth ES, Schuster SM: Arginine 30 and asparagine 74 have functional roles in the glutamine dependent activities of Escherichia coli asparagine synthetase B. J Biol Chem 269: 26789–26795 (1994).
Brears T, Liu C, Knight TJ, Coruzzi GM: Ectopic overexpression of asparagine synthetase in transgenic tobacco. Plant Physiol 103: 1285–1290 (1993).
Bullock WO, Fernandez JM, Short JM: High efficiency plasmid transforming recA Escherichia coli strain with beta galactosidase selection. Biotechniques 72: 376–378 (1987).
Cedar H, Schwartz JH: The asparagine synthetase of Escherichia coli J Biol Chem 244: 4112–4121 (1969).
Connell C, Fung S, Heiner C, Bridgham J, Chakerian V, Heron E, Jones B, Menchen S, Mordan W, Raff M, Recknor M, Smith L, Springer J, Woo S, Hunkapillar M: Automated DNA sequence analysis. Biotechniques 5: 342–348 (1987).
Davies KM, King GA: Isolation and characterization of a cDNA clone for a harvest-induced asparagine synthetase from Asparagus officinalis L. Plant Physiol 102: 1337–1340 (1993).
Denhardt DT: A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun 23: 641 (1966).
Erlich H: PCR Technology: Principles and Applications for DNA Amplification, Stockton Press, New York (1989).
Feinberg AP, Vogelstein B: Atechnique for radiolabeling DNA restriction enzyme fragments to high specific activity. Anal Biochem 132: 6–12 (1983).
Felton J, Michaelis S, Wright A: Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bact 142: 221–228 (1980).
Feng D-F, Doolittle RF: Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol 25: 351–360 (1987).
Fowden L, Lea PJ: Asparagine metabolism in higher plants. Biochem Physiol Pflanzen 168S: 3–14 (1975).
Frohman MA, Dusk MK, Martin GR: Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA 85: 8998 (1988).
Hongo S, Fujimori M, Shioda S, Nakai Y, Takeda M, Sato T: Immunochemical characterization of rat testicular asparagine synthetase. Arch Biochem Biophys 295: 120–125 (1992).
Hongo S, Sato T: Some molecular properties of asparagine synthetase from rat liver. Biochim Biophys Acta 742: 484–489 (1983).
Horowitz B, Meister A: Glutamine-dependent asparagine synthetase from leukemia cells: chloride dependence, mechanism of action and inhibition. J Biol Chem 247: 6708–6719 (1972).
Huber TA, Streeter JG: Purification and properties of asparagine synthetase from soybean root nodules. Plant Sci 42: 9–17 (1985).
Huber TA, Streeter JG: Asparagine biosynthesis in soybean nodules. Plant Physiol 74: 605–610 (1984).
Hubert R, Simoni RD: Genetic and biochemical studies demonstrating a second gene coding for asparagine synthetase in Escherichia coli. J Bact 142: 212–220 (1980).
Joshi CP: Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucl Acids Res 15: 9624–9640 (1987).
Joy KW, Ireland RJ, Lea PJ: Asparagine synthesis in pea leaves and the occurrence of an asparagine synthetase inhibitor. Plant Physiol 73: 165–168 (1983).
Kalinski A, Melroy DL, Dwivedi RS, Herman EM: A soybean vacuolar protein (P34) related to thiol proteases is synthesized as a glycoprotein precursor during seed maturation. J Biol Chem 267: 12068–12067 (1992).
Kimura M: The Neutral Theory of Molecular Evolution. Cambridge University Press, Cambridge, UK (1983).
Lam HM, Peng SS, Coruzzi GM: Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol 106: 1347–1357 (1994).
Lea PJ, Fowden L: The purification and properties of glutamine-dependent asparagine synthetase isolated from Lupinus albus. Proc Ray Soc Lond Ser B 192: 13–26 (1975).
Luehr CA, Schuster SM: Purification and characterization of beef pancreatic asparagine synthetase. Arch Biochem Biophys 237: 335–346 (1985).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Mei B, Zalkin H: A cysteine-histidine-aspartate triad is involved glutamine amide transfer functionin purF-type glutamine amidotransferases. J Biol Chem 264: 16613–16619 (1989).
Michaels SD, John MC, Amasino RM: Removal of polysaccharides from plant DNA by ethanol precipitation. Biotechniques 17: 275–276 (1994).
Nakamura M, Yamada M, Hirota Y, Sugimoto K, Oka A, Takanami M: Nucleotide sequence of the asnA gene coding for asparagine synthetase in E. coli K-12. Nucl Acids Res 9: 4669–4676 (1981).
Needleman SB, Wunsch CP: A general method application to the search for similarities in the amino acid sequences of two proteins. J Mol Biol 48: 435–453 (1970).
Rognes SE: Anion regulation of lupin asparagine synthetase: chloride activation of glutamine utilizing reaction. Phytochemistry 19: 2287–2293 (1980).
Rognes SE: Glutamine-dependent asparagine synthetase from Lupinus lutus. Phytochemistry 14: 1975–1982 (1975).
Rognes SE: A glutamine-dependent asparagine synthetase from yellow lupine seedlings. FEB Lett 10: 62–66 (1970).
Scofield MA, Lewis WS, Schuster SM: Nucleotide sequence of Escherichia coli asnB and deduced amino acid sequence of asparagine synthetase B. J Biol Chem 265: 12895–12902 (1990).
Sheng S, Moraga D, Van Heeke G, Schuster S: High-level expression of human asparagine synthetase and production of monoclonal antibodies for enzyme purification. Protein Expr Purif 3: 337–346 (1992).
Sieciechowicz KA, Joy KW, Ireland RJ: The metabolism of asparagine in plants. Phytochemistry 27: 663–671 (1988).
Streeter JG: Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol 60: 235–239 (1977).
Streeter JG: In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys 157: 613–624 (1973).
Stulen I, Israelstam GF, Oaks A: Enzymes of asparagine synthesis in maize roots. Planta 146: 237–241 (1979).
Ta TC, MacDowall FDH, Faris MA: Asparagine synthetase from root nodules of alfalfa. Biochem Cell Biol 67: 455–459 (1989).
Tsai FY, Coruzzi GM: Light represses transcription of asparagine synthetase genes in photosynthetic and nonphotosynthetic organs of plants. Mol Cell Biol 11: 4966–4972 (1991).
Tsai FY, Coruzzi GM: Dark-induced and organ-specific expression of two asparagine synthetase genes in Pisum sativum. EMBO J 9: 323–332 (1990).
Van Heeke G, Schuster SM: Expression of human asparagine synthetase in Saccharomyces cerevisiae. Protein Engin 3: 739–744 (1990).
Van Heeke G, Schuster SM: Expression of human asparagine synthetase in Escherichia coli. J Biol Chem 264: 5503–5509 (1989).
Van Heeke G, Schuster SM: The N-terminal cysteine of human asparagine synthetase is essential for glutamine binding activity. J Biol Chem 264: 19475–19477 (1989).
Zalkin H: The amidotransferases. Adv Enzymol Relat Areas Mol Biol 66: 203–309 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hughes, C.A., Beard, H.S. & Matthews, B.F. Molecular cloning and expression of two cDNAs encoding asparagine synthetase in soybean. Plant Mol Biol 33, 301–311 (1997). https://doi.org/10.1023/A:1005784202450
Issue Date:
DOI: https://doi.org/10.1023/A:1005784202450