Abstract
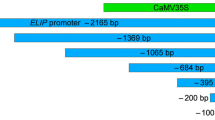
Two lipoxygenase (LOX) genes (tomloxA and tomloxB) are expressed in ripening tomato fruit, and tomloxA is also expressed in germinating seedlings [12]. The 5'-upstream regions of these genes were isolated to study the regulatory elements involved in coordinating tomlox gene expression. Sequence analysis of the promoters did not reveal any previously characterized regulatory elements except for TATA and CAAT boxes. However, the sequence motif GATAcAnnAAtnTGATG was found in both promoters. Chimeric gene fusions of each tomlox promoter with the β-glucuronidase reporter gene (gus) were introduced into tobacco and tomato plants via Agrobacterium-mediated transformation. GUS activity in tomloxA-gus plants during seed germination peaked at day 5 and was enhanced by methyl jasmonate (MeJa) treatment. No GUS activity was detected in tomloxB-gus seedlings. Neither wounding nor abscisic acid (ABA) treatment of transgenic seedlings modified the activity of either promoter. During fruit development, GUS expression in tomloxA-gus tobacco fruit increased 5 days after anthesis (DAA) and peaked at 20 DAA. In tomloxB-gus tobacco fruit, GUS activity increased at 10 DAA and peaked at 20 DAA. In transgenic tomato fruit, tomloxA-gus expression was localized to the outer pericarp during fruit ripening, while tomloxB-gus expression was localized in the outer pericarp and columella. These data demonstrate that the promoter regions used in these experiments contain cis-acting regulatory elements required for proper regulation of tomlox expression during development and for MeJa-responsiveness.
Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K: Short Protocols in Molecular Biology, 2nd ed. Green Publishing Associates and John Wiley and Sons, New York (1992).
Bell E, Mullet JE: Lipoxygenase gene expression is modulated in plants by water deficit, wounding, and methyl jasmonate. Mol Gen Genet 230: 456–462 (1991).
Bell E, Mullet JE: Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103: 1133–1137 (1993).
Berestetzky V, Dathe W, Daletskaya T, Musatenko L, Seinbdner G: Jasmonic acid in seed dormancy of Acer ataricum. Biochem Physiol Pflanzen 187: 13–19 (1991).
Bonnet J-L, Crouzet J: Lipoxygenase from tomato fruit: partial purification and study of some properties. J Food Sci 42: 625–627 (1977).
Bowsher CG, Ferrie BJM, Ghosh S, Todd J, Thompson JE, Rothstein SJ: Purification and partial characterization of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol 100: 1802–1807 (1992).
Bunker TW, Koetje DS, Stephenson LC, Creelman RA, Mullet JE, Grimes HD: Sink limitation induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. Plant Cell 7: 1319–1331 (1995).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Croft KPC, Voisey CR, Slusarenko AJ: Mechanism of hypersensitive cell collapse: correlation of increased lipoxygenase activity with membrane damage in leaves of Phaseolus vulgaris (L.) inoculated with an avirulent race of Pseudomonas syringae pv. phaseolicola. Physiol Mol Plant Path 36: 49–62 (1990).
Eiben HG, Slusarenko A: Complex spatial and temporal expression of lipoxygenase during Phaseolus vulgaris (L.) development. Plant J 5: 123–135 (1994).
Farmer EE, Ryan CA: Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 (1992).
Ferrie BJ, Beaudoin N, Burkhart W, Bowsher CG, Rothstein SJ: The cloning of two tomato lipoxygenase genes and their differential expression during fruit ripening. Plant Physiol 106: 109–118 (1994).
Feys M, Naesens W, Tobback P, Maes E: Lipoxygenase activity in apples in relation to storage and physiological disorders. Phytochemistry 19: 1009–1011 (1980).
Fillati JA, Kiser J, Rose R, Comai L: Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Bio/technology 5: 726–730 (1987).
Franceschi VR, Grimes HD: Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci USA 88: 6745–6749 (1991).
Galliard T, Matthew JA: Lipoxygenase-mediated cleavage of fatty acids to carbonyl fragments in tomato fruits. Phytochemistry 16: 339–343 (1977).
Geerts A, Feltkamp D, Rosahl S: Expression of lipoxygenase in wounded tubers of Solanum tuberosum L. Plant Physiol 105: 269–277 (1994).
Giraudat J, Parcy F, Bertauche N, Gosti F, Leung J, Morris P-C, Bouvier-Durand M, Vartanian N: Current advances in abscisic acid action and signalling. Plant Mol Biol 26: 1557–1577 (1994).
Grierson C, Du J-S, de Torres Zabala M, Beggs K, Smith C, Holdsworth M, Bevan M: Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J 5: 815–826 (1994).
Grimes HD, Koetje DS, Franceschi VR: Expression, activity, and cellular accumulation of methyl jasmonate-responsive lipoxygenase in soybean seedlings. Plant Physiol 100: 433–443 (1992).
Hatanaka A, Kajiwara T, Matsui K, Kitamura A: Expression of lipoxygenase and hydroperoxide lyase activities in tomato fruits. Z Naturforsch 47c: 369–374 (1992).
Hildebrand DF, Hamilton-Kemp TR, Legg CS, Bookjans G: Plant lipoxygenases: occurrence, properties and possible functions. Curr Top Plant Biochem Physiol 7: 201–219 (1988).
Hildebrand DF, Rodriguez JG, Legg CS, Brown GC, Bookjans G: The effects of wounding and mite infestation on soybean leaf lipoxygenase levels. Z Naturforsch 44c: 655–659 (1989).
Izawa T, Foster R, Nakajima M, Shimamoto K, Chua N-H: The rice bZIP transcriptional activator RITA-1 is highly expressed during seed development. Plant Cell 6: 1277–1287 (1994).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions:β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Kato T, Shirano Y, Iwamoto H, Shibata D: Soybean lipoxygenase L-4, a component of the 94-kilodalton storage protein in vegetative tissues: expression and accumulation in leaves induced by pod removal and by methyl jasmonate. Plant Cell Physiol 34: 1063–1072 (1993).
Keppler LD, Novacky A: The initiation of membrane lipid peroxidation during bacteria-induced hypersensitive reaction. Physiol Mol Plant Path 30: 233–245 (1987).
Kim S-R, Choi J-L, Costa MA, An G: Identification of G-box sequence as an essential element formethyl jasmonate response of potato proteinase inhibitor II promoter. Plant Physiol 99: 627–631 (1992).
Koch E, Meier BM, Eiben H-G, Slusarenko A: A lipoxygenase from leaves of tomato (Lycopersicon esculentum Mill.) is induced in response to plant pathogenic pseudomonads. Plant Physiol 99: 571–576 (1992).
Kosugi S, Ohashi Y, Nakajima K, Arai Y: An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70: 133–140 (1990).
Legge RL, Cheng K-H, Lepock JR, Thompson JE: Differential effects of senescence on the molecular organization of membranes in ripening tomato fruit. Plant Physiol 81: 954–959 (1986).
Lester G: Lipoxygenase activity of hypodermal-and middlemesocarp tissues from netted muskmelon fruit during maturation and storage. J Am Soc Hort Sci 115: 612–615 (1990).
Mason HS, DeWald DB, Mullet JE: Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell 5: 241–251 (1993).
Mattanovich D, Rüker F, da Câmara Machado A, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H: Efficient transformation of Agrobacterium spp. by electroporation. Nucl Acids Res 17: 6747 (1989).
Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK: An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol 101: 441–450 (1993).
Michelet B, Lukaszewicz M, Dupriez V, Boutry M: A plant plasma membrane proton-ATPase gene is regulated by development and environment and shows signs of a translational regulation. Plant Cell 6: 1375–1389 (1994).
Montgomery J, Pollard V, Deikman J, Fischer RL: Positive and negative regulatory regions control the spatial distribution of polygalacturonase transcription in tomato fruit pericarp. Plant Cell 5: 1049-1062 (1993).
Ocampo CA, Moerschbacher B, Grambow HJ: Increased lipoxygenase activity is involved in the hypersensitive response of wheat leaf cells infected with avirulent rust fungi or treated with fungal elicitor. Z Naturforsch 41c: 559–563 (1986).
Ohta H, Shida K, Peng Y-L, Furusawa I, Shishiyama J, Aibara S, Morita Y: A lipoxygenase pathway is activated in rice after infection with the rice blast fungus Magnaporthe grisea. Plant Physiol 97: 94–98 (1991).
Park TK, Holland MA, Laskey JG, Polacco JC: Germinationassociated lipoxygenase transcripts persist in maturing soybean plants and are induced by jasmonate. Plant Sci 96: 109–117 (1994).
Park TK, Polacco, JC: Distinct lipoxygenase species appear in the hypocotyl/radicle of germinating soybean. Plant Physiol 90: 285–290 (1989).
Rastogi R, Dulson J, Rothstein SJ: Cloning of tomato (Lycopersicon esculentum Mill.) arginine decarboxylase gene and its expression during fruit ripening. Plant Physiol 103: 829–834 (1993).
Ravnikar M, Vilhar B, Gogala N: Stimulatory effects of jasmenic acid on potato stem node and protoplast culture. J Plant Growth Regul 11: 29–33 (1992).
Rothstein SJ, Lahners KN, Lotstein RJ, Carozzi NB, Jayne SM, Rice DA: Promoter cassettes, antibiotic-resistance genes, and vectors for plant transformation. Gene 53: 153–161 (1987).
Saravitz DM, Siedow JN: The lipoxygenase isozymes in soybean [Glycine max (L.) Merr.] leaves: changes during leaf development, after wounding, and following reproductive sink removal. Plant Physiol 107: 535–543 (1995).
Shen Q, Ho T-HD: Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABAresponsive complexes each containing a G-box and a novel cis-acting element. Plant Cell 7: 295–307 (1995).
Siedow JN: Plant lipoxygenase: structure and function. Annu Rev Plant Physiol Plant Mol Biol 42: 145–188 (1991).
Thompson JE: The molecular basis for membrane deterioration during senescence. In: Noodén LD, Leopold AC (eds), Senescence and Aging in Plants, pp. 51–83. Academic Press, San Diego, pp. 51–83 (1988).
Todd JF, Paliyath G, Thompson JE: Characteristics of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol 94: 1225–1232 (1990).
Tranbarger TJ, Franceschi VR, Hildebrand DF, Grimes HD: The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3: 973–987 (1991).
Uknes S, Dincher S, Friedrich L, Negrotto D, Williams S, Thompson-Taylor H, Potter S, Ward E, Ryals J: Regulation of pathogenesis-related protein-1a gene expression in tobacco. Plant Cell 5: 159–169 (1993).
Vick BA, Zimmerman DC: Oxidative systems for modification of fatty acids: the lipoxygenase pathway. In: Stumpf PK, Conn EE (eds) The Biochemistry of Plants, vol. 9, pp. 53–90, Academic Press, New York (1987).
Yu LM, Lamb CJ, Dixon RA: Purification and biochemical characterization of proteins which bind to the H-box cis-element implicated in transcriptional activation of plant defense genes. Plant J 3: 805–816 (1993).
Zamora R, Olias JM, Mesias JL: Purification and characterization of tomato lipoxygenase. Phytochemistry 26: 345–347 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beaudoin, N., Rothstein*, S.J. Developmental regulation of two tomato lipoxygenase promoters in transgenic tobacco and tomato. Plant Mol Biol 33, 835–846 (1997). https://doi.org/10.1023/A:1005773722657
Issue Date:
DOI: https://doi.org/10.1023/A:1005773722657