Abstract
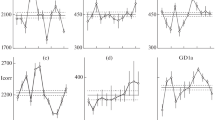
Glycolipid compositions of the human glioma cell lineT98G were studied during each phase of thecell cycle to see if those cell surfacemolecules are concerned with cell proliferation. In vitrocultured non-synchronized T98G cells are composed of ceramidemonohexoside(CMH), ceramidedihexoside (CDH), ceramidetrihexoside (CTH) and neolactotetraosylceramide (nLc4Cer)as neutral glycolipids, and of sulfatide (CS), gangliosidesGM3, GM2, GD1a and several other gangliosides asacidic ones. While total glycolipid content per cellularweight was shown to be increased during theM phase, deletion of complex gangliosides particularly b-seriesgangliosides was recognized (p < 0.05). The glycolipidprofile in other phases was fairly consistent, andthere was no glycolipid molecule specific to acertain phase of the cell cycle. Relative enhancementof simple gangliosides with a decrease of complexones during mitotic division may imply the functionalinvolvement of complex gangliosides in cell-cell or cell-matrixattachment, which may have to be abandoned duringthe process of detachment from the matrix orcellular cleavage.
Similar content being viewed by others
References
Hakomori S-I: Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Ann Rev Biochem 50: 733–764, 1981
Obeid LM, Linardic CM, Karolak LA, Hannun YA: Programmed cell death induced by ceramide. Science 259: 1769–1771, 1993
Hakomori S-I: Cell density-dependent changes of glycolipid concentrations in fibroblasts, and loss of their response in virus-transformed cells. Proc Natl Acad Sci USA 67: 1741–1747, 1970
Wolf BA, Robbins PW: Cell mitotic cycles synthesis of NIL hamster glycolipids including the Forssman antigen. J Cell Biol 61: 676–687, 1974
Hakomori S-I: Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res 45: 2405–2414, 1985
Chatterjee S, Sweeley CC, Velicer LF: Glycosphingolipids of human KB cells grown in monolayer, suspension, and synchronized cultures. J Biol Chem 250: 61–66, 1975
Lingwood CA, Hakomori S-I: Selective inhibition of cell growth and associated changes in glycolipid metabolism induced by monovalent antibodies to glycolipids. Exp Cell Res 108: 385–391, 1977
Chatterjee S, Velicer LF, Sweeley CC: Glycosphingolipid glycosyl hydrolases and glycosidases of synchronized human KB cells. J Biol Chem 250: 4972–4979, 1975
Scheideler MA, Lockney MW, Dawson G: Cell-cycle dependence of a ganglioside glycosyltransferase activity and its inhibition by enkephalin in a neurotumor cell line. J Neurochem 42: 1175–1182, 1984
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD: Molecular Biology of the Cell, Garland Publishing Inc., New York, 727–790, 1989
Bootsma D, Budke L, Vos O: Studies on synchronous division of tissue culture cells initiated by excess thymidine. Exp Cell Res 33: 301–309, 1964
Pfeiffer SE, Tolmach LJ: Inhibition of DNA synthesis in HeLa cells by hydroxyurea. Cancer Res 27: 124–129, 1967
Terashima T, Tolmach LJ: Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res 30: 344–362, 1963
Choi BO, Yamaki T, Ibayashi Y, Maeda Y, Gasa S, Hashi K: Interleukin 4 enhances ganglioside GD3 expression on the human fibroblast cell line WI-38. J Biochem 117: 315–320, 1995
Higashi H, Fukui Y, Ueda S, Kato S, Hirabayashi Y, Matsumoto M, Naiki M: Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem 95: 1517–1520, 1984
Yamakawa T, Irie R, Iwanaga M: The chemistry of lipid of posthemolytic residue or stroma of erythrocytes. IX. Sialic acid chromatography of mammalian stroma glycolipids. J Biochem 48: 490–507, 1960
Watanabe I, Okada S: Effects of temperature on growth rate of cultured mammalian cells (L5178Y). J Cell Biol 32: 309–323, 1967
Rösner H, Greis CH, Rodemann HP: Density-dependent expression of ganglioside GM3 by human skin fibroblasts in all-or-none fashion, as a possible modulator of cell growth in vitro. Exp Cell Res 190: 161–169, 1990
Lucocq JM, Berger EG, Warren G: Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol 109: 463–474, 1989
Cikes M, Friberg S Jr: Expression of H-2 and Moloney leukemia virus-determined cell-surface antigens in synchronized cultures of a mouse cell line. Proc Natl Acad Sci USA 68: 566–569, 1971
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choi, B.O., Yamaki, T., Tatewaki, K. et al. Deletion of complex gangliosides of human glioma cells during mitotic cell division. J Neurooncol 34, 211–219 (1997). https://doi.org/10.1023/A:1005742716197
Issue Date:
DOI: https://doi.org/10.1023/A:1005742716197