Abstract
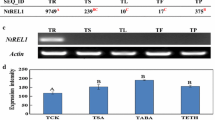
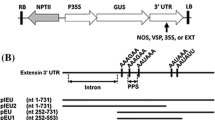
A genomic clone (Ext 1.4) encoding an extensin was isolated from a Nicotiana tabacum genomic library. The encoded polypeptide showed features characteristic of extensins such as Ser-(Pro)4 repeats and a high content in Tyr and Lys residues. The presence of one Tyr-Leu-Tyr-Lys motif suggests the possibility for one intramolecular isodityrosine cross-link whereas numerous Val-Tyr-Lys motifs may participate in intermolecular cross-links. This extensin appears to be close to an extensin already characterized in N. tabacum but very different from the Ext 1.2 extensin of N. sylvestris. The analysis of genomic DNA gel blots using probes spanning different parts of the gene showed that the Ext 1.4 gene belongs to a complex multigene family having one member originating from N. sylvestris and three members from N. tomentosiformis. The Ext 1.4 specific probe found a 1.4 kb mRNA in stems, roots, ovaries and germinating seeds of healthy plants. The Ext 1.4 gene family is strongly induced in actively dividing cell suspension cultures and after wounding of leaves or stems in conditions where root formation occurs. On the contrary, it is not induced in leaves in response to a hyperensitive reaction to a viral infection or after elicitor treatment.
Similar content being viewed by others
References
Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S: A new elicitor of the hypersensitive response to tobacco: a fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J 8: 551–560 (1995).
Baldwin TC, Coen ES, Dickinson HG: The ptl1 gene expressed in the transmitting tissue of Antirrhinum encodes an extension-like rotein. Plant J 2: 733–739 (1992).
Bradley DJ, Kjelbom P, Lamb CJ: Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30 (1992).
Brownleader MD, Dey PM: Purification of extensin from cell walls of tomato (hybrid of Lycopersicon esculentum and L. peruvianum) cells in suspension culture. Planta 191: 457–469 (1992).
Carpita NC, Gibeaut DM: Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 (1993).
Cassab GI, Varner JE: Cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39: 321–353 (1986).
Chen J, Varner JE: An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J 4: 2145–2155 (1985).
Chen CG, Cornish EC, AE Clarke: Specific expression of an extensin-like gene in the style of Nicotiana alata. Plant Cell 4: 1053–1062 (1992).
Chen CG, Mau SL, Clarke AE: Nucleotide sequence and style-specific expression of a novel proline-rich protein gene from Nicotiana alata. Plant Mol Biol 21: 391–395 (1993).
Cooper JB, Heuser JE, Varner JE: 3,4-Dehydroproline inhibits cell wall assembly and cell division in tobacco protoplasts. Plant Physiol 104: 747–752 (1994).
Corbin DR, Sauer N, Lamb CJ: Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol 7: 4337–4344 (1987).
Criqui MC, Plesse B, Durr A, Marbach J, Parmentier Y, Jamet E, Fleck J: Characterization of genes expressed in mesophyll protoplasts of Nicotiana sylvestris before the re-initiation of the DNA replicational activity. Mech Devel 38: 121–132 (1992).
De Loose M, Tire GG, Villaroel R, Genetello C, Van Montagu M, Depicker A, Inzé D: The extensin signal peptide allows secretion of a heterologous protein from protoplasts. Gene 99: 95–100 (1991).
Drews GN, Beals TP, Bui AQ, Goldberg RB: Regional and cell-specific gene expression patterns during petal development. Plant Cell 4: 1383–1404 (1992).
Epstein L, Lamport DTA: An intramolecular linkage involving isodityrosine in extensin. Phytochemistry 23: 1241–1246 (1984).
Evans IM, Gatehouse LN, Gatehouse JA, Yarwood JN, Boulter D, Croy RRD: The extensin gene family in oilseed rape (Brassica napus L.): characterization of sequences of representative members of the family. Mol Gen Genet 223: 273–287 (1990).
Feinberg AP, Vogelstein B: A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Genschik P, Criqui MC, Parmentier Y, Marbach J, Durr A, Fleck J, Jamet E: Isolation and characterization of a cDNA encoding a 3-hydroxy-3-methylglutaryl coenzyme A reductase from Nicotiana sylvestris. Plant Mol Biol 20: 337–341 (1992).
Goldman MH de S, Pezzotti M, Seurinck J, Mariani C: Developmental expression of tobacco pistil-specific genes encoding novel extensin-like proteins. Plant Cell 4: 1041–1051 (1992).
Goodspeed TH: The Genus Nicotiana: Origins, Relationships and Evolution of its Species in the Light of their Distribution, Morphology and Cytogenetics. Chronica Botanica, Waltham, MA (1954).
Hong JC, Cheong YH, Nagao RT, Bahk JD, Cho MJ, Key JL: Isolation and characterization of three soybean extensin cDNAs. Plant Physiol 104: 793–796 (1994).
Jamet E, Durr A, Parmentier Y, Criqui MC, Fleck J: Is ubiquitin involved in the dedifferentiation of higher plant cells? Cell Diff Devel 29: 37–46 (1990).
Jamet E, Parmentier Y, Durr A, Fleck J: Genes encoding the small subunit of RUBISCO belong to two highly conserved subfamilies in Nicotianeae. J Mol Evol 33: 226–236 (1991).
Kawalleck P, Schmeltzer E, Hahlbrock K, Somssich IE: Two pathogen-responsive genes in parsley encode a tyrosine-rich hydroxyproline-rich glycoprotein (HRGP) and an anionic peroxidase. Mol Gen Genet 24: 444–452 (1995).
Kawasaki S: Extensin secreted into the culture medium of tobacco cells. II. Extensin subfamilies of similar composition. Plant Cell Physiol 32: 721–728 (1991).
Keller B, Lamb CJ: Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Devel 3: 1639–1646 (1989).
Kieliszewski MJ, Lamport DTA: Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172 (1994).
Lind JL, Bacic A, Clarke AE, Anderson MA: A style-specific hydroxyproline-rich glycoprotein with properties of both extensins and arabinogalactan proteins. Plant J 6: 491–502 (1994).
Ludevid MD, Ruiz-Avila L, Vallès MP, Stiefel V, Torrent M, Torné JM, Puigdomenech P: Expression of genes for cell-wall proteins in dividing and wounded tissues of Zea mays L. Planta 180: 524–529 (1990).
Maliga P, Breznovits AS, Marton L: Streptomycin-resistant plants from callus culture of haploid tobacco. Nature New Biol 244: 29–30 (1973).
Malik AN, McLean PM, Roberts A, Barnett PS, Demaine AG, Banga JP, McGregor AM: A simple high yield method for the preparation of lambda gt10 DNA suitable for subcloning, amplification and direct sequencing. Nucl Acids Res 13: 4031–4032 (1990).
Marty I, Brugidou C, Chartier Y, Meyer Y: Growth-related gene expression in Nicotiana tabacum mesophyll protoplasts. Plant J 4: 265–278 (1993).
Meeks-Wagner DRy, Dennis ES, Tran Thanh Van K, Peacock J Tobacco genes expressed during in vitro floral initiation and their expression during normal plant development. Plant Cell 1: 25–35 (1989).
Memelink J, Swords KMM, de Kam RJ, Schilperoort RA, Hoge JHC, Staehelin LA: Structure and regulation of tobacco extensin. Plant J 4: 1011–1022 (1993).
Meyer Y, Abel WO: Importance of the wall for cell division and in the activity of the cytoplasm in cultured tobacco protoplasts. Planta 123: 33–40 (1975).
Meyer Y, Chartier Y, Grosset J, Marty I, Brugidou C, Marinho P, Rivera-Madrid R: Gene expression in mesophyll protoplasts. In: Roubelakis-Angelakis KA, Tran Thanh van K (eds) Plant Morphogenesis: Molecular Approaches. NATO ASI series 253: 221–236 (1993).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 (1962).
Nagata T, Ishida S, Hasezawa S, Takahashi Y: Genes involved in the dedifferentiation of plant cells. Int J Devel Biol 38: 321–327 (1994).
Nagy JJ, Maliga P: Callus induction and plant regeneration from mesophyll protoplasts of Nicotiana sylvestris. Z Pflanzenphysiol 78: 453–455 (1976).
Niebel A, de Almeida Engler J, Tiré C, Engler G, Van Montagu M, Gheysen G: Induction patterns of an extensin gene in tobacco upon nematode infection. Plant Cell 5: 1697–1710 (1993).
Okamuro JK, Goldberg RB: Tobacco single-copy DNA is highly homologous to sequences present in the genomes of its diploid progenitors. Mol Gen Genet 198: 290–298 (1985).
Parmentier Y, Durr A, Marbach J, Hirsinger C, Criqui MC, Fleck J, Jamet E: A novel wound-inducible extensin gene is expressed early in newly isolated protoplasts of Nicotiana sylvestris. Plant Mol Biol 29: 279–292 (1995).
Qi X, Behrens BX, West PR, Mort AJ: Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures. Plant Physiol 108: 1691–1701 (1995).
Raz R, José M, Moya A, Martinez-Izquierdo JA, Puigdomenech P: Different mechanisms generating sequence variability are revealed in distinct regions of the hydroxyproline-rich glycoprotein gene from maize and related species. Mol Gen Genet 233: 252–259 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schilde-Rentschler L: Role of the cell wall in the ability of tobacco protoplasts to form callus. Planta 135: 177–181 (1977).
Schmidt JS, Lindstrom JT, Vodkin LO: Genetic length polymorphism create size variation in proline-rich proteins of the cell wall. Plant J 6: 177–186 (1994).
Schnabelrauch LS, Kieliszewski M, Upham BL, Alizedeh H, Lamport DTA: Isolation of pI 4.6 extensin from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J 9: 477–489 (1996).
Showalter AM: Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 (1993).
Showalter AM, Bell JN, Cramer CL, Bailey JA, Varner JE: Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci USA 82: 6551–6555 (1985).
Showalter AM, Zhou J, Rumeau D, Worst SG, Varner JE: Tomato extensin and extensin-like cDNAs: structure and expression in response to wounding. Plant Mol Biol 16: 547–565 (1991).
Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B: Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie 75: 687–706 (1993).
Tagu D, Walker N, Ruiz-Avila L, Burgess S, Martinez-Izquierdo JA, Leguay JJ, Netter P, Puigdomènech P: Regulation of the maize HRGP gene expression by ethylene and wounding. mRNA accumulation and qualitative expression analysis by microprojectile bombardment. Plant Mol Biol 20: 529–538 (1992).
Tiré C, de Rycke R, De Loose M, Inze D, Van Montagu M, Engler G: Extensin gene expression is induced by mechanical stimuli leading to local cell wall strengthening in Nicotiana plumbaginifolia. Planta 195: 195: 175–181 (1994).
van Regenmortel: Tobamoviruses. In: Kurstak E (ed) Handbook of Plant Virus Infections and Comparative Diagnosis, pp. 541–564 Elsevier/North Holland Medical Press, Amsterdam (1981).
Varner JE, Lin LS: Plant cell wall architecture. Cell 56: 231–239 (1989).
Vera P, Lamb C, Doerner PW: Cell cycle regulation of hydroxyproline-rich glycoprotein HRGPnt3 gene expression during the initiation of lateral root meristems. Plant J 6: 717–727 (1994).
von Heijne G: Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem 133: 17–21 (1983).
Wilson LG, Fry JC: Extensin - a major cell wall glycoprotein. Plant Cell Environ 9: 239–260 (1986).
Ye ZH, Song YR, Marcus A, Varner JE: Comparative localization of three classes of cell wall proteins. Plant J 1: 175–183 (1991).
Zhou J, Rumeau D, Showalter AM: Isolation and characterization of two wound-regulated tomato extensin genes. Plant Mol Biol 20: 5–17 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirsinger, C., Parmentier, Y., Durr, A. et al. Characterization of a tobacco extensin gene and regulation of its gene family in healthy plants and under various stress conditions. Plant Mol Biol 33, 279–289 (1997). https://doi.org/10.1023/A:1005738815383
Issue Date:
DOI: https://doi.org/10.1023/A:1005738815383