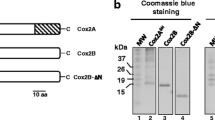
Abstract
The branched respiratory chain of the archaeon Sulfolobus acidocaldarius contains a supercomplex, SoxM, consisting of a bc1-like subcomplex and a terminal oxidase moiety, including a subunit II analogous polypeptide, SoxH. However, the latter component has never been identified in preparations of SoxM. We demonstrate the presence of an mRNA transcript by Northern analysis. We succeeded in cloning and expressing the respective gene with truncated N-terminus by deleting a 20 AS membrane anchor, which resulted in a water-soluble purple copper protein, which was further characterized. The recombinant subunit II of the SoxM complex contains a correctly inserted binuclear CuA cluster as revealed by UV/vis and EPR spectroscopy. The protein is highly thermostable and displays a redox potential of +237 mV. In recombinant form, the metal interacts with cytochrome c as an artificial electron donor; the physiological electron donor is still unknown, since S. acidocaldarius does not contain any c-type cytochromes. The purple copper center of SoxM shows an interesting pH dependency with a pKa at 6.4, suggesting protonation of the Cu-ligating histidines. Further lowering the pH causes a reversible transition into another cluster form with concomitant liberation of one copper. It may thus provide a model for the study of cluster rearrangements in response to pH.
Similar content being viewed by others
REFERENCES
Brock, T. D., Brock, K. M., Belly, R. T., and Weiss, R. L. (1972). Arch. Microbiol. 84, 54–68.
Castresana, J., Lübben, M., and Saraste, M. (1995). J. Mol. Biol. 250, 202–210.
Farrar, J. A., Neese, F., Lappalainen, P., Kroneck, P. M. H., Saraste, M., Zumft, W. G., and Thomson, A. J. (1996). J. Amer. Chem. Soc. 118, 11501–11514.
Gleissner, M., Elferink, M. G. L., Driessen, A. J. M., Konings, W. N., Anemüller, S., and Schäfer, G. (1994). Eur. J. Biochem. 224, 983–990.
Gleissner, M., Kaiser, U., Antonopuolos, E., and Schäfer, G. (1997). J. Biol. Chem. 272, 8417–8426.
Harlowe, E. and Lane, D. (1988). Antibodies, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
Hendriks, J., Gohlke, U., and Saraste, M. (1998). J. Bioenerg. Biomembr. 30, 15–24.
Hettmann, T., Schmidt, C. L., Anemuller, S., Zähringer, U., Moll, H., Petersen, A., and Schafer, G. (1998). J. Biol. Chem. 273, 12032–12040.
Iwata, S., Ostermeier, C., Ludwig, B., and Michel, H. (1995). Nature (London) 376, 660–669.
Kim, R., Sandler, S. J., Goldman, S., Yokota, H., and Clark, A. J. (1998). Biotechnol. Lett. 20, 207–210.
Lappalainen, P., Aasa, R., Malmström, B. G., and Saraste, M. (1993). J. Biol. Chem. 268, 26416–26421.
Lübben, M., Arnaud, S., Castresana, J., Warne, A., Albracht, S. P. J., and Saraste, M. (1994). Eur. J. Biochem. 224, 151–159.
Michel, H., Behr, J., Harrenga, A., and Kannt, A. (1998). Annu. Rev. Biophys. Biomol. Struct. 27, 329–356.
Schäfer, G., Engelhard, M., and Müller, V. (1999). Microbiol. Molbiol. Rev. 63, 570–620.
Slutter, C. E., Sanders, D., Wittung, P., Malmström, B. G., Aasa, R., Richards, J. H., Gray, H. B., and Fee, J. A. (1996). Biochemistry 35, 3387–3395.
Trumpower, B. L. and Gennis, R. B. (1994). Annu. Rev. Biochem. 63, 675–716.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1995). Science 269, 1069–1074.
Van der Oost, J., De Boer, A. P. N., De Gier, J.-W. L., Zumft, W. G., Stouthamer, A. H., and Van Spanning, R. J. M. (1994). FEMS Microbiol. Lett. 121, 1–10.
van Miltenburg, R., Ruger, B., Grunewald-Janho, S., Leons, M., and Schroder, C. (1995). The DIG System User's Guide for Filter Hybridization, Laboratory Manual, Roche-Diagnostics.
Williams, P. A., Blackburn, N. J., Sanders, D., Bellamy, H., Stura, E. A., Fee, J. A., and McRee, D. E. (1999). Nature Struct. Biol. 6, 509–516.
Wilmanns, M., Lappalainen, P., Kelly, M., Sauer-Eriksson, E., and Saraste, M. (1995). Proc. Natl. Acad. Sci. USA 92, 11955–11959.
Rights and permissions
About this article
Cite this article
Komorowski, L., Anemüller, S. & Schäfer, G. First Expression and Characterization of a Recombinant CuA-Containing Subunit II from an Archaeal Terminal Oxidase Complex. J Bioenerg Biomembr 33, 27–34 (2001). https://doi.org/10.1023/A:1005668522801
Issue Date:
DOI: https://doi.org/10.1023/A:1005668522801