Abstract
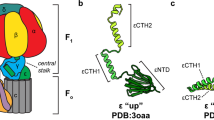
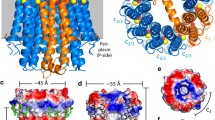
The ATP synthase from Escherichia coli is a prototype of the ATP synthases that are found in many bacteria, in the mitochondria of eukaryotes, and in the chloroplasts of plants. It contains eight different types of subunits that have traditionally been divided into F1, a water-soluble catalytic sector, and Fo, a membrane-bound ion transporting sector. In the current rotary model for ATP synthesis, the subunits can be divided into rotor and stator subunits. Several lines of evidence indicate that ε is one of the three rotor subunits, which rotate through 360 degrees. The three-dimensional structure of ε is known and its interactions with other subunits have been explored by several approaches. In light of recent work by our group and that of others, the role of ε in the ATP synthase from E. coli is discussed.
Similar content being viewed by others
REFERENCES
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994). Nature (London) 370, 621-628.
Adachi, K., Yasuda, R., Noji, H., Itoh, H., Harada, Y., Yoshida, M., and Kinosita, K. Jr. (2000). Proc. Natl. Acad. Sci. USA 97, 7243-7247.
Aggeler, R., and Capaldi, R. A. (1996). J. Biol. Chem. 271, 13888-13891.
Aggeler, R., Chicas-Cruz, K., Cai, S. X., Keana, J. F., and Capaldi, R. A. (1992). Biochemistry 31, 2956-61.
Aggeler, R., Haughton, M. A., and Capaldi, R. A. (1995). J. Biol. Chem. 270, 9185-9191.
Allison, W. S. (1998). Acc. Chem. Res. 31, 819-826.
Altendorf, K., Stalz, W., Greie, J., and Deckers-Hebestreit, G. (2000). J. Exp. Biol. 203, 19-28.
Bianchet, M. A., Hullihen, J., Pedersen, P. L., and Amzel, L. M. (1998). Proc. Natl. Acad. Sci. USA 95, 11065-11070.
Birkenhager, R., Greie, J. C., Altendorf, K., and Deckers-Hebestreit, G. (1999). Eur. J. Biochem. 264, 385-396.
Boyer, P. D. (1997). Annu. Rev. Biochem. 66, 717-749.
Buckley, N. M., Cruz, J. A., Cohen, W. S., and McCarty, R. E. (1999). Photosynth. Res. 59, 137-145.
Bulygin, V. V., Duncan, T. M., and Cross, R. L. (1998). J. Biol. Chem. 273, 31765-31769.
Capaldi, R. A., and Schulenberg, B. (2000). Biochim. Biophys. Acta 1458, 263-269.
Cherepanov, D. A., Mulkidjanian, A. Y., and Junge, W. (1999). FEBS Lett. 449, 1-6.
Dallmann, H. G., Flynn, T. G., and Dunn, S. D. (1992). J. Biol. Chem. 267, 18953-60.
Dmitriev, O., Jones, P. C., Jiang, W. P., and Fillingame, R. H. (1999a). J. Biol. Chem. 274, 15598-15604.
Dmitriev, O. Y., Jones, P. C., and Fillingame, R. H. (1999b). Proc. Natl. Acad. Sci. USA 96, 7785-7790.
Dunn, S. D. (1982). J. Biol. Chem. 257, 7354-7359.
Dunn, S. D., Zardorozny, V. D., Tozer, R. G., and Orr, L. E. (1987). Biochemistry 26, 4488-4493.
Dunn, S. D., Tozer, R. G., and Zadorozny, V. D. (1990). Biochemistry 29, 4335-4340.
Elston, T., Wang, H. Y., and Oster, G. (1998). Nature (London) 391, 510-513.
Fillingame, R. H., Jones, P. C., Jiang, W., Valiyaveetil, F. I., and Dmitriev, O. Y. (1998). Biochim. Biophys. Acta 1365, 135-142.
Foster, D. L., and Fillingame, R. H. (1982). J. Biol. Chem. 257, 2009-2015.
Gardner, J. L., and Cain, B. D. (1999). Arch. Biochem. Biophys. 361, 302-308.
Grüber, G., and Capaldi, R. A. (1996). Biochemistry 35, 3875-3879.
Häsler, K., Engelbrecht, S., and Junge, W. (1998). FEBS Lett. 426, 301-304.
Häsler, K., Pänke, O., and Junge, W. (1999). Biochemistry 38, 13759-13765.
Hausrath, A. C., Grüber, G., Matthews, B.W., and Capaldi, R. A. (1999). Proc. Natl. Acad. Sci. USA 96, 13697-13702.
Hermolin, J., Dmitriev, O. Y., Zhang, Y., and Fillingame, R. H. (1999). J. Biol. Chem. 274, 17011-17016.
Jäger, H., Birkenhäger, R., Stalz, W. D., Altendorf, K., and Deckers-Hebestreit, G. (1998). Euro. J. Biochem. 251, 122-132.
Jiang, W. P., and Fillingame, R. H. (1998). Proc. Natl. Acad. Sci. USA 95, 6607-6612.
Jones, P. C., and Fillingame, R. H. (1998). J. Biol. Chem. 273, 29701-29705.
Junge, W. (1999). Proc. Natl. Acad. Sci. USA 96, 4735-4737.
Kaim, G., Matthey, U., and Dimroth, P. (1998). EMBO J. 17, 688-695.
Kato-Yamada, Y., Noji, H., Yasuda, R., Kinosita, K., Jr., and Yoshida, M. (1998). J. Biol. Chem. 273, 19375-19377.
Kato-Yamada, Bald, Y., D., Koike, M., Motohashi, K., Hisabori, T., and Yoshida, M. (1999). J. Biol. Chem. 274, 33991-33994.
Ketchum, C. J., and Nakamoto, R. K. (1998). J. Biol. Chem. 273, 22292-22297.
Kuki, M., Noumi, T., Maeda, M., Amemura, A., and Futai, M. (1988). J. Biol. Chem. 263, 17437-17442.
Laget, P. P., and Smith, J. B. (1979). Arch. Biochem. Biophys. 197, 83-89.
Long, J. C., Wang, S., and Vik, S. B. (1998). J. Biol. Chem. 273, 16235-16240.
Lötscher, H. R., deJong, C., and Capaldi, R. A. (1984a). Biochemistry 23, 4134-4140.
Lötscher, H. R., deJong, C., and Capaldi, R. A. (1984b). Biochemistry 23, 4140-4143.
McLachlin, D. T., Bestard, J. A., and Dunn, S. D. (1998). J. Biol. Chem. 273, 15162-15168.
Mendel-Hartvig, J., and Capaldi, R. A. (1991a). Biochemistry 30, 1278-1284.
Mendel-Hartvig, J., and Capaldi, R. A. (1991b). Biochemistry 30, 10987-10991.
Nakamoto, R. K., Ketchum, C. J., and Al-Shawi, M. K. (1999). Annu. Rev. Biophys. Biomol. Struct 28, 205-234.
Nakamoto, R. K., Ketchum, C. J., Kuo, P. H., Peskova, Y. B., and Al-Shawi, M. K. (2000). Biochim. Biophys. Acta 1458, 289-299.
Ogilvie, I., Aggeler, R., and Capaldi, R. A. (1997). J. Biol. Chem. 272, 16652-16656.
Oster, G., and Wang, H. (2000). Biochim. Biophys. Acta 1458, 482-510.
Pänke, O., Gumbiowski, K., Junge, W., and Engelbrecht, S. (2000). FEBS Lett. 472, 34-38.
Pänke, O., and Rumberg, B. (1999). Biochim. Biophys. Acta 1412, 118-128.
Rastogi, V. K., and Girvin, M. E. (1999). Nature (London) 402, 263-268.
Rodgers, A. J.W., and Capaldi, R. A. (1998). J. Biol. Chem. 273, 29406-29410.
Sabbert, D., and Junge, W. (1996). Proc. Natl. Acad. Sci. USA 94, 2312-2317.
Sambongi, Y., Iko, Y., Tanabe, M., Omote, H., Iwamoto-Kihara, A., Ueda, I., Yanagida, T., Wada, Y., and Futai, M. (1999). Science 286, 1722-1724.
Sawada, K., Kuroda, N., Watanabe, H., Moritani-Otsuka, C., and Kanazawa, H. (1997a). J. Biol. Chem. 272, 30047-30053.
Sawada, K., Watanabe, H., Moritani-Otsuka, C., and Kanazawa, H. (1997b). Arch. Biochem. Biophys. 348, 183-189.
Schulenberg, B., and Capaldi, R. A. (1999). J. Biol. Chem. 274, 28351-28355.
Schulenberg, B., Wellmer, F., Lill, H., Junge, W., and Engelbrecht, S. (1997). Eur. J. Biochem. 249, 134-141.
Schulenberg, B., Aggeler, R., Murray, J., and Capaldi, R. A. (1999). J. Biol. Chem. 274, 34233-34237.
Smith, J. B., and Sternweiss, P. C. (1977). Biochemistry 16, 306-311.
Sorgen, P. L., Bubb, M. R., and Cain, B. D. (1999). J. Biol. Chem. 274, 36261-36266.
Sorgen, P. L., Caviston, T. L., Perry, R. C., and Cain, B. D. (1998). J. Biol. Chem. 273, 27873-27878.
Stock, D., Leslie, A. G.W., and Walker, J. E. (1999). Science 286, 1700-1705.
Tang, C. L., and Capaldi, R. A. (1996). J. Biol. Chem. 271, 3018-3024.
Uhlin, U., Cox, G. B., and Guss, J. M. (1997). Structure 5, 1219-1230.
Valiyaveetil, F. I., and Fillingame, R. H. (1998). J. Biol. Chem. 273, 16241-16247.
Watts, S. D., and Capaldi, R. A. (1997). J. Biol. Chem. 272, 15065-15068.
Weber, J., and Senior, A. E. (1997). Biochim. Biophys Acta 1319, 19-58.
Wilkens, S., and Capaldi, R. A. (1998). J. Biol. Chem. 273, 26645-26651.
Wilkens, S., Dahlquist, F. W., McIntosh, L. P., Donaldson, L. W., and Capaldi, R. A. (1995). Natur. Struct. Biol. 2, 961-967.
Xiong, H., and Vik, S. B. (1995). J. Biol. Chem. 270, 23300-23304.
Xiong, H., Zhang, D., and Vik, S. B. (1998). Biochemistry 37, 16423-16429.
Yasuda, R., Noji, H., Kinosita, K., Jr., and Yoshida, M. (1998). Cell 93, 1117-1124.
Zhang, Y., and Fillingame, R. H. (1995). J. Biol. Chem. 270, 24609-24614.
Zhang, Y., Oldenburg, M., and Fillingame, R. H. (1994). J. Biol. Chem. 269, 10221-4.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vik, S.B. What Is the Role of ε in the Escherichia coli ATP Synthase?. J Bioenerg Biomembr 32, 485–491 (2000). https://doi.org/10.1023/A:1005664908066
Issue Date:
DOI: https://doi.org/10.1023/A:1005664908066