Abstract
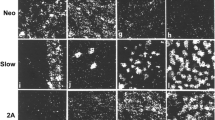
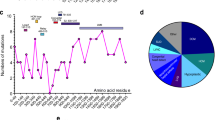
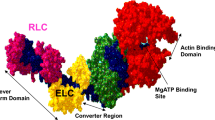
Myosin heavy chain (MyHC) is the major contractile protein of muscle. We report the first complete cosmid cloning and definitive physical map of the tandemly linked human skeletal MyHC genes at 17p13.1. The map provides new information on the order, size, and relative spacing of the genes, and it resolves uncertainties about the two fastest twitch isoforms. The physical order of the genes is demonstrated to contrast with the temporal order of their developmental expression. Furthermore, nucleotide sequence comparisons allow an approximation of the relative timing of five ancestral duplications that created distinct genes for the six isoforms. A firm foundation is provided for molecular analysis in patients with suspected primary skeletal myosinopathies and for detailed modelling of the hypervariable surface loops which dictate myosin's kinetic properties.
Similar content being viewed by others
References
Acakpo-Satchivi L, Edelmann W, Sartorius C, Lu B, Wahr P, Watkins S, Metzger J, Leinwand L and Kucherlapati R (1997) Growth and muscle defects in mice lacking adult myosin heavy chain genes. J Cell Biol 139: 1219–1229.
Argov Z, Gardner-Medwin D, Johnson M and Mastaglia F (1984) Patterns of muscle fiber-type disproportion in hypotonic infants. Arch Neurol 41: 53–57.
Asmussen G, Beckers-Bleukx G and Marechal G (1994) The force-velocity relation of the rabbit inferior oblique muscle; infuence of temperature. Pflugers Arch 426: 542–547.
Bar A and Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. Fed Eur Biochem Soc Letters 235: 153–155.
Barany M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50: (Suppl.) 197–218.
Bottinelli R, Canepari C, Reggiani C and Steinen G (1994) Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. J Physiol 481(3): 663–675.
Cooper S and Eccles J (1930) The isometric responses of mammalian muscles. J Physiol 69: 377–385.
Cope M, Whisstock J, Rayment I and Kendrick-Jones J (1996) Conservation within the myosin motor domain: implications for structure and function. Structure 4: 969–987.
Cripps R, Suggs J and Bernstein S (1999) Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J 18: 1793–1804.
Davis RW, Thomas M, Cameron J, St. John TP, Scherer S and Padgett RA (1980) Rapid DNA isolation for enzymatic and hybridization analysis. Meth Enzymol 65: 404–411.
Dominguez R, Freyzon Y, Trybus K and Cohen C (1998) Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 94: 559–571.
Epp T, Wang R, Sole M and Liew C (1995) Concerted evolution of mammalian cardiac myosin heavy chain genes. J Mol Evol 41: 284–292.
Fananapazir L, Dalakas M, Cyran F, Cohn G and Epstein N (1993) Missense mutations in the beta-myosin heavy-chain gene cause central core disease in hypertrophic cardiomyopathy. Proc Natl Acad Sci 90: 3993–3997.
Fuchs A and Luschei E (1971) Development of isometric tension in simian extraocular muscle. J Physiol 219: 155–166.
Furch M, Geeves M and Manstein D (1998) Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry 37: 6317–6326.
Geisterfer-Lowrance A, Christe M, Conner D, Ingwall J, Schoen F, Seidman C and Seidman J (1996) A mouse model of familial hypertrophic cardiomyopathy. Science 272: 731–734.
Geisterfer-Lowrance A, Kass S, Tanigawa G, Vosberg H, McKenna W, Seidman C and Seidman J (1990) A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62: 999–1006.
Green ED and Olsen MV (1990) Chromosomal region of the cystic fibrosis gene in yeast artificial chromosomes: a model for human gene mapping. Science 250: 94–98.
Grunstein M and Hogness DJ (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72: 3961–3965.
J, Hewett T, Klevitsky R, Buck S, Moss R and Robbins J (1997) Transgenic remodelling of the regulatory myosin light chains in the mammalian heart. Circ Res 80: 655–664.
Hastings GA and Emerson CPJ (1991) Myosin functional domains encoded by alternative exons are expressed in specific thoracic muscles of Drosophila. J Cell Biol 114: 263–276.
Jarcho J, McKenna W, Pare J, Solomon S, Holcombe R, Dickie S, Levi T, Donis-Keller H, Seidman J and Seidman C (1989) Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. New Eng J Med 321(20): 1372–1378.
Jullien EH, Kelly AM, Pompidou AJ, Hoffman R, Pompidou A, Schiaffino S, Stedman HH and Rubinstein NA (1995) Characterization of the human perinatal myosin heavy chain transcript. Eur J Biochem 230: 1001–1006.
Kelley C, Takahashi M, Yu J and Adelstein R (1993) An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 268: 12848–12854.
Kinose F, Wang S, Kidambi U, Moncman C and Winkelmann D (1996) Glycine 699 is pivotal for the motor activity of skeletal muscle myosin. J Cell Biol 134: 895–909.
Knotts S, Rindt H and Robbins J (1995) Postion independent expression and developmental regulation is directed by the beta myosin heavy chain gene's 5′ upstream region in transgenic mice. Nucl Acids Res 23: 3301–3309.
Laing N, Wilton S, Akkari P, Dorosz S, Boundy K, Kneebone C, Blumbergs P, White S, Watkins H and Love D (1995) A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nature Genetics 9: 75–79.
Larsson L and Moss R (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol (Lond) 472: 595–614.
Loke J and MacLennan D (1998) Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med 104: 470–486.
Mahdavi V, Strehler EE, Periasamy M, Wieczorek D, Izumo S, Grund S, Strehler MA and et al. (1986) Sarcomeric myosin heavy chain gene family: organization and pattern of expression. In: Emerson C, Fischman DA, Nadal-Ginard B, Siddique MA. Molecular Biology of Muscle Development. (pp. 345–361) Alan R. Liss, New York.
Mullis K, Faloona F, Scharf S, Saiki R, Horn G and Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol 51: 263–273.
Murphy C and Spudich J (1998) Dictyostelium myosin 25–50K loop substitutions specifically affect ADP release rates. Biochemistry 37: 6738–6744.
Nathans J (1994) In the eye of the beholder: visual pigments and inherited variation in human vision. Cell 78: 357–360.
Nowak KJ, Wattanasirichaigoon D, Goebel HH, Wilce M, Pelin K, Donner K, Jacob RL, Hubner C, Oexle K, Anderson JR, Verity CM, North KN, Iannaccone ST, Muller CR, Nurnberg P, Muntoni F, Sewry C, Hughes I, Sutphen R, Lacson AG, Swoboda KJ, Vigneron J, Wallgren-Pettersson C, Beggs A H and Laing NG (1999) Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 23: 208–212.
Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, de la Chapelle A and Wallgren-Pettersson C (1999) Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 96: 2305–2310.
Pelin K, Ridanpaa M, Donner K, Wilton S, Krishnarajah J, Laing N, Kolmerer B, Millevoi S, Labeit S, de la Chapelle A and Wallgren-Petterson C (1997) Refined localisation of the genes for nebulin and titin on chromosome 2q allows the assignment of nebulin as a candidate gene for autosomal recessive nemaline myopathy. Eur J Human Genet 5: 229–234.
Pette D and Staron R (1997) Mammalian skeletal muscle fiber type transitions. Int Rev Cytol 170: 143–223.
Rayment I, Rypiewski W, Schmidt-Base K, and et al. (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261: 50–58.
Riethman H, Spais C, Buckingham J, Grady D and Moyzis R (1993) Physical analysis of the terminal 240 kb of DNA from human chromosome 7q. Genomics 17: 25–32.
Rome L, Sosnicki A and Goble D (1990) Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol 431: 173–185.
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K and et al. (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibers. J Muscle Res Cell Motil 10: 197–205.
Schiaffino S and Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Reviews 76: 371–422.
Schwartz K, Lecarpentier Y, Martin J, Lompre A, Mercadier J and Swynghedauw B (1981) Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol 13: 1071–1075.
Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L and Schiaffino S (1994) Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiology 267(6 Pt 1): C1723–1728.
Spudich J (1994) How molecular motors work. Nature 372: 515–518.
Stedman HH, Eller M, Jullian EH, Fertels SH, Sarkar S, Sylvester JE, Kelly AM and Rubinstein NA (1990) The human embryonic myosin heavy chain: complete primary structure reveals evolutionary relationships with other developmental isoforms. J Biol Chem 265: 3568–3576.
Strehler EE, Strehler-Page MA, Perriard JC, Periasamy M and Nadal-Ginard BJ (1986) Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene: evidence against intron-dependent evolution of the rod. J Mol Biol 190: 291–317.
Sweeney H, Rosenfeld S, Brown F, Faust L, Smith J, Xing J, Stein L and Sellers J (1998) Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem 273: 6262–6270.
Tanigawa G, Jarcho J, Kass S, Solomon S, Vosberg H, Seidman J and Seidman C (1990) A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell 62: 991–998.
Uyeda TQP, Ruppel KM and Spudich JA (1994) Enzymatic activities correlate with chimaeric substitutions at the actin-binding face of myosin. Nature 368: 567–569.
Vale R (1996) Switches, latches, and amplifiers: common themes of G proteins and molecular motors. J Cell Biol 135: 291–302.
Wahl GM, Lewis KA, Ruiz JC, Rothernberg B, Zhao J and Evans GA (1987) Cosmid vectors for rapid genomic walking, restriction mapping, and gene transfer. Proc Natl Acad Sci USA 84: 2160–2164.
Weiss A, McDonough D, Wertman B, Acakpo-Satchivil L, Montgomery K, Kucherlapati R, Leinwand L, and Krauter K (1999) Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. Proc Natl Acad Sci USA 96: 2958–2963.
Wieczorek D, Periasamy M, Butler-Browne G, Whalen R and Nadal-Ginard B (1985) Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular muscle. J Cell Biol 101: 618–629.
Zhan W, Watchko J, Prakash Y and Sieck G (1998) Isotonic contractile and fatigue properties of developing rat diaphragm muscle. J Appl Physiol 84: 1260–1268.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shrager, J.B., Desjardins, P.R., Burkman, J.M. et al. Human skeletal myosin heavy chain genes are tightly linked in the order embryonic-IIa-IId/x-IIb-perinatal-extraocular. J Muscle Res Cell Motil 21, 345–355 (2000). https://doi.org/10.1023/A:1005635030494
Issue Date:
DOI: https://doi.org/10.1023/A:1005635030494