Abstract
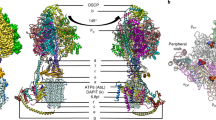
The structural and functional connection between the peripheral catalytic F1 sector and theproton-translocating membrane sector F0 of the mitochondrial ATP synthase is reviewed. Theobservations examined show that the N-terminus of subunit γ, the carboxy-terminal and centralregion of F0I-PVP(b), OSCP, and part of subunit d constitute a continuous structure, the lateralstalk, which connects the peripheries of F1 to F0 and surrounds the central element of thestalk, constituted by subunits γ and δ. The ATPase inhibitor protein (IF1) binds at one sideof the F1F0 connection. The carboxy-terminal segment of IF1 apparently binds to OSCP. The42L-58K segment of IF1, which is per se the most active domain of the protein, binds at thesurface of one of the three α/β pairs of F1, thus preventing the cyclic interconversion of thecatalytic sites required for ATP hydrolysis.
Similar content being viewed by others
REFERENCES
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994). Nature (London) 370, 621–628.
Aggeler, R., Ogilvie, I., and Capaldi, R. A. (1997). J. Biol. Chem. 272, 19621–19624.
Arnold, I., Pfeiffer, K., Neupert, W., Stuart, R. A., and Shagger, H. (1998). EMBO J. 17, 7170–7178.
Baracca, A., Barogi, S., Carelli, V., Lenaz, G., and Solaini, G. (2000). J. Biol. Chem. 275, 4177–4182.
Belogrudov, G. I., Tomich, J. M., and Hatefi, Y. (1996). J. Biol. Chem. 271, 20340–20345.
Birkenhager, R., Hoppert, M., Deckers-Hebestreit, G., Mayer, F., and Altendorf, K. (1995). Eur. J. Biochem. 230, 58–67.
Boekema, E. J., Van Breemen, J. F. L., Brisson, A., Ubbink-Kok, T., Konings, W. N., and Lolkema, J. S. (1999). Nature (London) 401, 37–38.
Bottcher, B., Schwarz, L., and Graber, P. (1998). J. Mol. Biol. 281, 757–762.
Boyer, P. D. (1997). Annu. Rev. Biochem. 66, 717–749.
Cabezon, E., Butler, P. J. G., Runswick, M. J., and Walker, J. E. (2000). J. Biol. Chem., 275, 25460–25464.
Capaldi, R. A., Aggeler, R., Turina, P., and Wilkens, S. (1994). Trends Biochem. Sci. 19, 284–289.
Capaldi, R. A., Schulenberg, B., Murray, J., and Aggeler, R. (2000). J. Exp. Biol. 203, 29–33.
Cherepanov, D. A., Mulkidjanian, A. Y., and Junge, W. (1999). FEBS Lett. 449, 1–6.
Collinson, I. R., van Raaij, M. J., Runswick, M. J., Fearnley, I. M., Skehel, J. M., Orris, G. L., Miroux, B., and Walker, J. E. (1994a). J. Mol. Biol. 242, 408–421.
Collinson, I. R., Fearnley, I. M., Skehel, J. M., Runswick, M. J., and Walker, J. E. (1994b). Biochem. J. 303, 639–645.
Collinson, I. R., Runswick, M. J., Buchanan, S. K., Fearnley, I. M., Skehel, J. M., van Raaij, M. J., Griffiths, D. E., and Walker, J. E. (1994c). Biochemistry 33, 7971–7978.
Cox, G. B., Devenish, R. J., Gibson, F., Howitt, S. M., and Nagley, P. (1992). In Molecular Mechanism in Bioenergetics (Ernster, L., ed.). Elsevier, Amsterdam, The Netherlands, pp. 283–315.
Duncan, T. M., Bulygin, V.V., Zhou, Y., Hutcheon, M. L., and Cross, R. L. (1995). Proc. Natl. Acad. Sci. USA 92, 10964–10968.
Dunn, S. D., Heppel, L. A., and Fullmer, C. S. (1980). J. Biol. Chem. 255, 6891–6896.
Elston, T., Wang, H., and Oster, G. (1998). Nature (London) 391, 510–513.
Engelbrecht, S. and Junge, W. (1997). FEBS Lett. 414, 485–491.
Fernandez-Moran, H. (1962). Circulation 26, 1039–1065.
Fillingame, R. H. (1996). Curr. Opin. Struct. Biol. 6, 491–498.
Fillingame, R. H. (1997). J. Exp. Biol. 200, 217–224.
Fillingame, R. H., Jones, P. C., Jiang, W., Valiyaveetil, F. I., and Dmitriev, O.Y. (1998). Biochim. Biophys. Acta 1365, 135–142.
Gaballo, A., Zanotti, F., Raho, G., and Papa, S. (1999). FEBS Lett. 463, 7–11.
Gaballo, A., Zanotti, F., Solimeo, A., and Papa S., (1998). Biochemistry 37, 17519–17526.
Garnier J., Gibrat, J. F., and Robson, B. (1996). Methods Enzymol. 266, 540–553.
Gogol, E. P., Lucken, U., and Capaldi, R. A. (1987). FEBS Lett. 219, 274–278.
Green, D. W. and Grover, G. J. (2000). Biochim. Biophys. Acta 1458, 343–355.
Guerrieri, F., Zanotti, F., Capozza, G., Colaianni, G., Ronchi, S., and Papa, S., (1991). Biochim. Biophys. Acta 1059, 348–354.
Harris, D. A. and Das, A. M. (1991). Biochem. J. 280, 561–573.
Hausrath, A. C., Gruber, G., Matthews, B. W., and Capaldi, R. A. (1999). Proc. Natl. Acad. Sci. USA 96, 13697–13702.
Heckman, C., Tomich, J. M., and Hatefi, Y. (1991). J. Biol. Chem. 266, 13564–13571.
Houstek, J., Kopecky, J., Zanotti, F., Guerrieri, F., Jirillo, E., Capozza, G., and Papa, S. (1988). Eur. J. Biochem. 173, 1–8.
Hundal, T., Norling, B., and Ernster, L. (1983). FEBS Lett. 162, 5–10.
Jackson, P. J. and Harris, D. A. (1988). FEBS Lett. 229, 224–228.
Joshi, S. and Burrows, R. (1990). J. Biol. Chem. 265, 14518–14525.
Junge, W., Lill, H., and Engelbrecht, S. (1997). Trends Biochem. Sci. 22, 123–126.
Karrasch, S. and Walker, J. E. (1999). J. Mol. Biol. 290, 379–384.
Kato-Yamada, Y., Noji, H., Yasuda, R., Kinosita, K., and Yoshida, M. (1998). J. Biol. Chem. 273, 19375–19377.
Lebowitz, M. S. and Pedersen, P. L. (1996). Azch. Biochem. Biophys. 330, 342–354.
Mendel-Hartvig, J. and Capaldi, R. A. (1991). Biochim. Biophys. Acta 1060, 115–124.
Mimura, H., Hashimoto, T., Yoshida, Y., Ichikawa, N., and Tagawa, K. (1993). J. Biochem. 113, 350–354.
Noji, H., Yasuda, R., Yoshida, M., and Kinosita, K. (1997). Nature (London) 386, 299–302.
Oda, T., Futaky, S., Kitagawa, K., Yoshihara, Y., Tani I., and Higuti, T. (1989). Biochem. Biophys. Res. Commun. 165, 449–456.
Ogilvie, I., Aggeler, R., and Capaldi, R. A. (1997). J. Biol. Chem. 272, 16652–16656.
Oster, G. and Wang, H. (1999). Structure 7, R 67-R72.
Ovchinnikov, Y. A., Modyanov, N. N., Grinkevich, V. A., Aldanova, N. A., Trubetskaya, O. E., Nazimov, I. V., Hundal, T., and Ernster, L. (1984). FEBS Lett. 166, 19–22.
Packer, L., Tristram, S., Herz, J. M., Russel, C., and Borders, C. L. (1979). FEBS Lett. 108, 243–248.
Panke, O., Gumbiowski, K., Junge, W., and Engelbrecht (2000). FEBS Lett. 472, 34–38.
Papa, S., Guerrieri, F., Zanotti, F., Houstek, J., Capozza, G., and Ronchi, S. (1989). FEBS Lett. 249, 62–66.
Papa, S., Zanotti, F., Cocco, T., Perrucci, C., Candita, C., and Minuto, M. (1996). Eur. J. Biochem. 240, 461–467.
Papa, S., Xu, T., Gaballo, A., and Zanotti, F. (1999). In Frontiers of Cellular Bioenergetics: Molecular Biology, Biochemistry and Physiopathology (Papa, S., Guerrieri, F., Tager, J. M., eds.), Plenum Press, London, New York, pp. 459–487.
Paul, M. F., Guerin, B., and Velours, J. (1992). Eur. J. Biochem. 205, 163–172.
Pedersen, P. L., Schwerzmann, K., and Cintron, N. M. (1981). Curr. Top. Bioenerge. 11, 149–199.
Raho, G., Zanotti, F., Gaballo, A., Vuolo, R., and Papa, S. (2000). In preparation.
Sabbert, D., Engelbrecht, S., and Junge, W. (1996). Nature (London) 381, 623–625.
Sambongi, Y., Iko, Y., Tanabe, M., Omote, H., Iwamoto-Kihara, A., Ueda, I., Yanagida, T., Wada, Y., and Futai, M. (1999). Science 286, 1722–1724.
Schneider, E. and Altendorf, K. (1984). Proc. Natl. Acad. Sci. USA 81, 7279–7283.
Schneider, E. and Altendorf, K. (1985). Eur. J. Biochem. 153, 105–109.
Schneider, E. and Altendorf, K. (1987). Microbiol. Rev. 51, 477–497.
Senior, A. E. (1990). Annu. Rev. Biophys. Biophys. Chem. 19, 7–41.
Singh, S., Turina, P., Bustamante, C. J., Keller, D. J., and Capaldi, R. A. (1996). FEBS Lett. 397, 30–34.
Stock, D., Leslie, A. G. W., and Walker, J. E. (1999). Science 286, 1700–1705.
Stout, J. S., Partridge, B. E., Dibbern, D. A., and Schuster, S. M. (1993). Biochemistry 32, 7496–7502.
Takeyasu, K., Omote, H., Nettikadan, S., Tokumasu, F., Iwamoto-Kihara, A., and Futai, M.(1996). FEBS Lett. 392, 110–113.
Tsunoda, S. P., Aggeler, R., Noji, H., Kinosita, K., Yoshida, M., and Capaldi, R. A. (2000). FEBS Lett. 470, 244–248.
Ubbink-Kok, T., Boekema, E. J., van Breemen, J. F., Brisson, A., Konings, W. N., and Lolkema, J. S. (2000). J. Mol. Biol. 296, 311–321.
van Raaij, M. J., Orris, G. L., Montgomery, M. G., Runswick, M. J., Fearnley, I. M., Skehel, J. M., and Walker, J. E. (1996). Biochemistry 35, 15618–15625.
Walker, J. E. and Collinson, I. R. (1994). FEBS Lett. 346, 39–43.
Walker, J. E., Fearnley, I. M., Gay, N. J., Gibson, B. W., Northrop, F. D., Powell, S. J., Runswick, M. J., Saraste, M., and Tybulewicz, W. L. J. (1985). J. Mol. Biol. 184, 677–701.
Walker, J. E., Runswick, M. J. and Poulter, L., (1987) J. Mol. Biol. 197, 89–100.
Watts, S. D., Zhang, Y., Fillingame, R. H., and Capaldi, R. A. (1995). FEBS Lett. 368, 235–238.
Watts, S. D., Tang, C., and Capaldi, R. A. (1996). J. Biol. Chem. 271, 28341–28347.
Wilkens, S. and Capaldi, R. A. (1998). Nature (London) 393, 29.
Wilkens, S., Dunn, S. D., Chandler, J., Dahlquist, F.W., and Capaldi, R. A. (1997). Nature Struct. Biol. 4, 198–201.
Wilkens, S., Zhou, J., Nakayama, J., Dunn, S. D., and Capaldi, R. (2000). J. Mol. Biol. 295, 387–391.
Xu, T., Candita, C., Amoruso, G., and Papa, S. (1998). Eur. J. Biochem. 252, 155–161.
Xu, T., Zanotti, F., Gaballo, A., Raho, G., and Papa, S. (2000). Eur. J. Biochem. 267, 4445–4455.
Zanotti, F., Guerrieri, F., Scarfó, R., Berden, J., and Papa, S. (1985). Biochem. Biophys. Res. Commun. 132, 985–990.
Zanotti, F., Guerrieri, F., Capozza, G., Houstek, J., Ronchi, S., and Papa, S. (1988). FEBS Lett. 237, 9–14.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Papa, S., Zanotti, F. & Gaballo, A. The Structural and Functional Connection between the Catalytic and Proton Translocating Sectors of the Mitochondrial F 1 F 0 -ATP Synthase . J Bioenerg Biomembr 32, 401–411 (2000). https://doi.org/10.1023/A:1005584221456
Issue Date:
DOI: https://doi.org/10.1023/A:1005584221456