Abstract
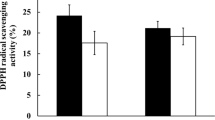
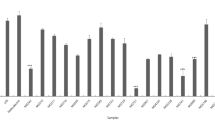
The antioxidative effect of intact cells and intracellular cell-free extracts of intestinal lactic acid bacteria B. longum (ATCC 15708) and L. acidophilus (ATCC 4356) was investigated. Both intact cells and intracellular cell-free extracts of 109 cells of B. longum and L. acidophilus demonstrated antioxidative activity, inhibiting linoleic acid peroxidation by 28–48%. This indicated that these two strains demonstrated excellent antioxidative activity. B. longum and L. acidophilus also showed the ability to scavenge α,α-diphenyl-β-picrylhydrazyl (DPPH) free radical, scavenging 21–52%. The intact cells of these two intestinal bacteria demonstrated a high inhibitory effect on the cytotoxicity of 4-nitroquinoline-N-oxide (4NQO). Cytotoxicity of 4NQO was reduced by L. acidophilus by approximately half and by almost 90% by B. longum. Nevertheless, no inhibition of cytoxicity observed for intracellular cell-free extracts of 109 cells of B. longum and L. acidophilus. The effect of B. longum and L. acidophilus on inhibiting plasma lipid peroxidation was also evaluated. The results showed that both intestinal strains were able to protect plasma lipid from oxidation at different degrees. The inhibition rates on plasma lipid peroxidation ranged from 11 to 29% for 109 cells of B. longum and L. acidophilus. Generally speaking, B. longum demonstrated better antioxidative ability than L. acidophilus in this study.
Similar content being viewed by others
REFERENCES
Fernandes CR, Shahani KM: Modulation of antibiosis by lactobacilli and yogurt and its healthful and beneficial significance. In Yogurt: Nutritional and Health Properties. RC Chandan, (ed), McLean, Virginia, National Yogurt Association, 1989
Gilliland SE: Health and nutritional benefits from lactic acid bacteria. FEMS Microbiol Rev 87:175–188, 1990
Lin MY: The beneficial effect of lactic acid bacteria. J Chin Nutr Soc 20:367–380, 1995
Lin MY, Yen CL: Antioxidative ability of lactic acid Bacteria. J Agric Food Chem 47:1460–1466, 1999
Hitchins AD, McDonough FE: Prophylactic and therapeutic aspects of fermented milk. Am J Clin Nutr 49:675–684, 1989
Sanders ME: Summary of conclusions from a consensus panel of experts on health attributes of lactic cultures: Significance to fluid milk products containing cultures. J Dairy Sci 76:1819–1828, 1993
Ahotupa M, Saxelin M, Korpela R: Antioxidative properties of Lactobacillus GG. Nutr Today (Suppl) 31:51S-52S, 1996
Kaizu H, Sasaki M, Nakajima H, Suzuki Y: Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 76:2493–2499, 1993
Korpela R, Peuhkuri K, Lahteenmaki T, Sievi E, Saxelin M, Vapaatalo H: Lactobacillus rhamnosus GG shows antioxidative properties in vascular endothelial cell culture. Milchwissenschaft 52:503–505, 1997
Sanders JW, Leehouts KJ, Haandrikmam AJ, Venema G, Kok J: Stress response in Lactococcus lactis: Cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J Bacteriol 177:5254–5260, 1995
Halliwell B: Free radicals and antioxidants: A personal view. Nutr Rev 52:253–265, 1994
Halliwell B, Chirico S: Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr 57:715S-725S, 1993
Halliwell B, Gutteridge JMC: Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–4, 1984
Wanasundrara U, Amarowicz R, Shanhidi F: Isolation and identification of an antioxidative component in canola meal. J Agric Food Chem 420:1285–1290, 1994
Decker E, Faraji H: Inhibition of lipid oxidation by carnosine. JAOCS 67:650–652, 1990
Shimada K, Fujikawa K, Yahara K, Nakamura T: Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948, 1992
Carmichael J, DeGraff WG, Gardar AF: Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 47:936–939, 1987
Crouch GD, Helman LJ: All-trans-retinioc acid inhibits the growth of human rhbdomyosarcoma cell lines. Cancer Res 51:4882–4886, 1991
Mosmann T: Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–60, 1983
Twentyman PR, Luscombe M: A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 56:279–275, 1987
Rodriguez-Martinez MA, Ruiz-Torres A: Homeostasis etween lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech Ageing Dev 66:213–222, 1992
Steel RGD, Torrie JH: Principles and Procedures of Statistics: A Biomedical Approach, 2nd ed., New York, McGraw-Hill, 1980
Bertelsen G, Christophersen C, Nielsen PH, Madsen HL, Stadel P: Chromatographic isolation of antioxidants guided by a methyl linoleate assay. J Agric Food Chem 43:1272–1275, 1995
Husain SR, Gllard J, Gllard P: α-Tocopherol prooxidant effect and malondialdehyde production. Am Oil Chem Soc, 64:109–111, 1987
Farmer EH, Bloomfield GF, Sundralingam A, Sutton DA: The course and mechanism of autoxidation reactions in olefinic and polyolefinic substances, including rubber. Trans Faraday Soc 38:348–356, 1942
Halliwell B, Gutteridge JMC: Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J 1:441–445, 1989
Yano T, Takahashi S, Ichikawa T: Active oxygen generated in the process of carcinogen metabolism can induce oxidative damage in nuclei. Res Commun Mol Pathos Pharmacol 87:367–370, 1995
Fronza G, Madzak C, Campomenosi P, Inga A, Iannone R, Abbondandolo A, Sarasin A: Mutation spectrum of 4–nitroquinoline-1–oxide damaged single-stranded shuttle vector DNA transfected into monkey cells. Mutat Res. 308:117–125, 1994
Goldstein LB, Bertels C, Davies JN: Reliability of the NIH stroke scale. Arch Neurol 46:660–662, 1989
Lai YC: Plasma levels of antioxidants and oxidative products in normal subjects and stroke patients and their relationship with early recovery of stroke. Master thesis. National Chung Hsing University, Taiwan, 1996
Kaizu H, Sasaki M, Nakajima H, and Suzuki Y: Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 76:2493–2499, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, MY., Chang, FJ. Antioxidative Effect of Intestinal Bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci 45, 1617–1622 (2000). https://doi.org/10.1023/A:1005577330695
Issue Date:
DOI: https://doi.org/10.1023/A:1005577330695