Abstract
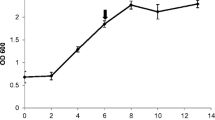
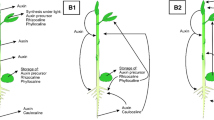
We examined several environmental and developmental influences on trypsin inhibitor (TI) activity in leaves of young Brassica napus seedlings in a series of greenhouse experiments. In seedlings of B. napus cv. Westar, TI activity is constitutively present and exhibits a rise then fall through time in the first true leaves of young plants. TI activity is induced by wounding in the first true leaves, but the degree of induction is relatively insensitive to the degree of wounding over a gradient of 5–15% of leaf area damage. TI activity is enhanced in first true leaves of plants in which the cotyledons have been wounded relative to plants in which the cotyledons have not been wounded. TI activity is also enhanced in the second true leaves on plants in which the first true leaves have been wounded. The degree of systemic induction in second true leaves declines additively with plant age, but local induction in the first true leaves is not affected by age. In B. napus cv. Gido, TI activity is constitutively present but is not locally wound-inducible in first true leaves of young plants exposed to the same wounding gradient as cv. Westar. In unwounded plants at the six-leaf stage, TI activity is higher in second true leaves than in fifth true leaves, indicating that TI activity is developmentally regulated in this cultivar.
Similar content being viewed by others
REFERENCES
Alarcon, J.-J., and Malone, M. 1995. The influence of plant age on wound induction of proteinase inhibitors in tomato. Physiol. Plant. 95:423–427.
Baldwin, I. T. 1999. Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. J. Chem. Ecol. 25:3–30.
Baldwin, I. T., and Schmelz, E. A. 1994. Constraints on an induced defense: The role of leaf area. Oecologia 97:424–430.
Bodnaryk, R. P. 1992. Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 31:2671–2677.
Bodnaryk, R. P. 1997. Will low glucosinolate cultivars of the mustards Brassica juncea and Sinapis alba be vulnerable to insect pests? Can. J. Plant. Sci. 77:283–287.
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
Broadway, R. M. 1989. Tryptic inhibitory activity in wild and cultivated crucifers. Phytochemistry 28:755–758.
Broadway, R. M. 1995. Are insects resistant to plant proteinase inhibitors? J. Insect Physiol. 41:107–116.
Broadway, R. M., and Duffey, S. S. 1986. Plant proteinase inhibitors: Mechanism of action and effect on the growth and digestive physiology of larval Heliothis zea and Spodoptera exigua. J. Insect Physiol. 32:827–833.
Broadway, R. M., and Missurelli, E. L. 1990. Regulatory mechanisms of tryptic inhibitory activity in cabbage plants. Phytochemistry 29:3721–3725.
Broadway, R. M., Duffey, S. S., Pearce, G., and Ryan, C. A. 1986. Plant proteinase inhibitors: A defense against insects? Entomol. Exp. App. 41:33–38.
Chew, F. S. 1988. Searching for defensive chemistry in the Cruciferae, or, do glucosinolates always control interactions of Cruciferae with their potential herbivores and symbionts? No! pp. 81–112, in K. C. Spencer (ed.). Chemical Mediation of Coevolution. Academic Press, San Diego.
Cipollini, D. F. 1998. Induced defenses and phenotypic plasticity. Trends Ecol. Evol. 13:200.
Dewitt, T. J., Sih, A., and Wilson, D. S. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13:77–81.
Domoney, C., Welham, T., Ellis, N., and Hellens, R. 1994. Inheritance of qualitative and quantitative trypsin inhibitor variants in Pisum. Theor. Appl. Genet. 89:387–391.
Duke, J. 1983. Handbook of Energy Crops. Unpublished. Purdue University Library.
Felton, G. W., Broadway, R. M., and Duffey, S. S. 1989. Inactivation of proteinase inhibitor activity by plant-derived quinones: Complications for host plant resistance against noctuid herbivores. J. Insect Physiol. 35:981–990.
Green, T. R., and Ryan, C. A. 1972. Wound-induced proteinase inhibitors in plant leaves: A possible defense mechanism against insects. Science 175:776–777.
Gustafson, G., and Ryan, C. A. 1976. The specificity of protein turnover in tomato leaves: The accumulation of proteinase inhibitors, induced with the wound hormone PIIF. J. Biol. Chem. 251:7004–7010.
Hopkins, R. J., Ekbom, B., and Henkow, L. 1998. Glucosinolate content and susceptibility for insect attack of three populations of Sinapis alba. J. Chem. Ecol. 24:1203–1216.
Johnson, R., Narvaez, J., An, G., and Ryan, C. A. 1990. Expression of proteinase inhibitors I and II in transgenic tobacco plants: Effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. U.S.A. 86:9871–9875.
Jongsma, M. A., and Bolter, C. 1997. The adaptation of insects to plant proteinase inhibitors. J. Insect Physiol. 43:885–895.
Jongsma, M. A., Baker, P. L., and Stiekema, W. J. 1993. Quantitative determination of serine proteinase inhibitor activity using a radial diffusion assay. Anal. Biochem. 212:79–84.
Jongsma, M. A., Bakker, P. L., Visser, B., and Stiekema, W. J. 1994. Trypsin inhibitor activity in mature tomato and tobacco plants is mainly induced locally in response to insect attack, wounding and virus infection. Planta 195:29–35.
Karban, R., and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago, Illinois.
Karban, R., Agrawal, A. A., and Mangel, M. 1997. The benefits of induced defenses against herbivores. Ecology 78:1351–1355.
Koiwa, H., Bressan, R. A., and Hasegawa, P. M. 1997. Regulation of proteinase inhibitors and plant defense. Trends Plant Sci. 2:379–384.
Kollipara, K. P., Singh, L., and Hymowitz, T. 1994. Genetic variation of trypsin and chymotrypsin inhibitors in pigeopea [Cajanus cajan ( L.) Millsp.] and its wild relatives. Theor. Appl. Genet. 88:986–993.
McManus, M. T., White, D. W. R., and McGregor, P. G. 1994. Accumulation of a chymotrypsin inhibitor in transgenic tobacco can affect the growth of insect pests. Trans. Res. 3:50–58.
Rosenthal, G. A., and Berenbaum, M. R. 1992. Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press, San Diego, California.
Thaler, J. S., Stout, M. J., Karban, R., and Duffey, S. S. 1996. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 22:1767–1781.
Van Dam, N. M., and Vrieling, K. 1994. Genetic variation in constitutive and inducible pyrrolizidine alkaloid levels in Cynoglossum officinale L. Oecologia 99:374–378.
Zangerl, A. R., and Berenbaum, M. R. 1990. Furanocoumarin induction in wild parsnip: Genetics and populational variation. Ecology 71:1933–1940.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cipollini, D.F., Bergelson, J. Environmental and Developmental Regulation of Trypsin Inhibitor Activity in Brassica napus. J Chem Ecol 26, 1411–1422 (2000). https://doi.org/10.1023/A:1005540107957
Issue Date:
DOI: https://doi.org/10.1023/A:1005540107957