Abstract
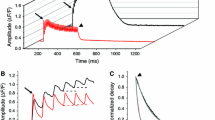
Fluorescence signals from the calcium sensitive dyes Fluo-3 or Rhod-2 were obtained simultaneously with isometric tension in single fibres isolated from the anterior tibialis muscle of Leptodactylus insularis (20–22°C). Fluo-3 fluorescence signals were transformed into [Ca2+]i transients as previously described. Most of the decay phase of single twitch transient is well fitted by a single exponential (τ of about 10 ms), followed by a slower declining component lasting tens of milliseconds. During short periods, 10 to 20 s, of low frequency stimulation, between 0.2 and 5 Hz, the basal [Ca2+]i increased slowly from 0.1 to about 0.4 μM, with only minor changes in the exponentially decaying phase. In fibres poisoned with thapsigargin or cyclopiazonic acid (1–2 μM) the τ of decay of fluorescence or Ca2+ transients of single twitches was very similar to that observed in non-poisoned fibres. Nevertheless, in poisoned fibres challenged with repetitive stimulation, the τ of Ca2+ transients decay increased from about 10 ms to > 40 ms, while the basal [Ca2+]i increased from 0.1 to 2 μM. Short rest periods (about 5 min) could reverse these effects, indicating that they were not a direct consequence of SR Ca2+-ATPase inhibition. The correlation coefficient between τ of decay and basal [Ca2+]i was > 0.8 (P < 0.0001). Qualitatively similar results were obtained measuring Rhod-2 fluorescence signals. A lumped, two-compartment model could account for these results. Loading the fibres with EGTA-AM, diminished the effects of prolonged stimulation observed in poisoned fibres. Moreover, we show that the Na+ – Ca2+ exchange mechanism does not participate appreciably in fast Ca2+ removal.
Similar content being viewed by others
References
Abbott BC (1951) The heat production associated with the mainte-nance of a prolonged contraction and the extra heat produced during large shortening. J Physiol Lond 112: 438-445.
Cannell MB and Allen DG (1984) Model of calcium movement during activation in the sarcomere of frog skeletal muscle. Biophys J 45: 913-925.
Cannell MB (1986) Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol Lond 376: 203-218.
Caputo C and Bolaños P (1978) Effect of external sodium and calcium on calcium e.ux in frog striated muscle. J Membr Biol 41: 1-14.
Caputo C and Bolaños P (1994) Fluo-3 signals associated with potassium contractures in single amphibian muscle fibres. J Physiol Lond. 481: 119-128.
Caputo C and Bolaños P (1995) Effect of thapsigargin on isometric tension and Fluo-3 calcium transients of amphibian skeletal muscle fibres. Biophys J 68: A313.
Caputo C, Bolaños P and Escobar AL (1997) Fast calcium clearance by parvalbumin during single twitches in skeletal muscle fibres. Biophys J 72: A274.
Caputo C, Edman KAP, Lou F and Sun Y-B (1994) Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator Fluo-3 in frog muscle fibres. J Physiol Lond 478: 137-148.
Carroll SL, Klein MG and Schneider MF (1997) Decay of calcium transients after electrical stimulation in rat fast-and slow-twitch skeletal muscle fibres. J Physiol Lond 501: 573-588.
Claflin DR, Stephenson DG, Morgan DL and Julian FJ (1994) The early phase of the decline of the intracellular Ca2+ transient is not affected by thapsigargin. Biophys J 66: A301.
Colquhoun D and Hawkes AG (1995) A Q-Matrix cookbook. In: Sakman B and Neher E (eds). Single Channel Recording (pp. 589-633). Plenum Press, New York.
Du GG, Ashley CC and Lea TJ (1994) Effects of thapsigargin and cyclopiazonic acid on the sarcoplasmic reticulum Ca2+ pump of skinned fibres from skeletal muscle. PfluÈgers Arch 429: 169-175.
Edman KAP, Sun Y-B, Caputo C and Lou F (1995) Effects of BAPTA on force and intracellular Ca2+ transient during twitch and tetanus of frog muscle fibres. Biophys J 68: A178.
Escobar AL, Velez P, Kim AM, Cifuentes F, Fill M and Vergara J (1998) Kinetic properties of calcium indicators: rapid transient response to flash photolysis. PfluÈgers Arch 434: 615-631.
Gerday C and Gillis JM (1976) The possible role of parvalbumin in the control of contraction. J Physiol Lond 258: 96P-97P.
Gerday C, Goffard P and Taylor SR (1991) Isolation and character-ization of parvalbumins from skeletal muscles of a tropical amphibian, Leptodactylus insularis. J Comp Physiol B 161: 475-481.
Gillis JM (1985) Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta 811: 97-114.
Gillis JM, Thomason D, Lefreve J and Kretsinger RH (1982) Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil 3: 377-398.
Harkins AB, Kurebayashi N and Baylor SM (1993) Resting myoplas-mic free calcium in frog skeletal muscle estimated with Fluo-3. Biophys J 65: 865-881.
Heizmann CW (1984) Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible role in mammalian cells. Experientia 40: 910-921.
Hidalgo C, Cifuentes F and Donoso P (1991) Sodium-calcium exchange in transverse tubules vesicles isolated from amphibian skeletal muscle. Ann NY Acad Sci 369: 483-487.
Hou T-T, Johnson JD and Rall JA (1991) Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol Lond 441: 285-304.
Hoya A and Venosa RA (1995) Characteristics of Na+-Ca2+ exchange in frog skeletal muscle. J Physiol Lond 486: 615-627.
Jiang Y, Johnson JD and Rall JA (1996) Parvalbumin relaxes frog skeletal muscle when sarcoplasmic Ca2+-ATPase inhibited. Am J Physiol 270: C411-C417.
Johnson JD, Jiang Y and Flynn M (1997). Modulation of Ca2+ transients and tension by intracellular EGTA in intact frog muscle fibres. Am J Physiol 272: C1437-C1444.
Klein MG, Kovacs L, Simon BJ and Schneider MF (1991) Decline of myoplasmic Ca2+, recovery of calcium release, and sarcoplasmic Ca2+ pump properties in frog skeletal muscle J Physiol Lond 414: 639-671.
Melzer W, Rios E and Schneider MF (1986). The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol Lond 372: 261-292.
Même W, Huchet-Cadiou C and Léoty C (1998) Cyclopiazonic acid-induced changes in the contraction and Ca2+ transient of frog fast-twitch skeletal muscle. Am J Physiol 274: C253-C261.
Minta AJP, Kao Y and Tsien RY (1989) Fluorescence indicators for cytosolic calcium based on rhodamine, and fluoresceine chromo-fores. J Biol Chem 264: 8171-8178.
Morgan DL, Claflin DR and Julian FJ (1997). The relationship between tension and slowly varying intracellular calcium concen-tration in intact frog skeletal muscle. J Physiol Lond 500: 177-192.
Múntner M, Kásaer L, Weber J and Berchtold MW (1995) Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc Nat Acad Sci USA 92: 6504-6508.
Permiakov EA, Ostrovsky AL and Kalinichenko LP (1987) Stopped-flow kinetic studies of Ca(II) and Mg(II) dissociation in cod parvalbumin and bovine-lactalbumin. Biophys Chem 28: 225-233.
Pizarro G, Csernoch L, Uribe I, Rodriguez M and Rios E (1991) The relationship between Qc and Ca release from the sarcoplasmic reticulum in skeletal muscle. J Gen Physiol 97: 913-947.
Rall JA (1996) Role of Parvalbumin in skeletal muscle relaxation. News Physiol Sci 11: 249-255.
Sun Y-B, Lou F and Edman KAP (1996). The relationship between the intracellular Ca2+ transient and the isometric force in frog muscle fibres. Exp Physiol 81: 711-724.
Wahar PA, Johnson JD and Rall JA (1993) The role of sarcoplasmic reticulum (SR) in frog skeletal muscle relaxation. Proceedings of the 32th Congress of the IUPS, Glasgow,30 September (Sunday p. 49).
Westerblad H and Allen DG (1996). The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydro-quinone. J Physiol Lond 474: 291-301.
Westerblad H and Allen DG (1996). Slowing of relaxation and [Ca2+]i during prolonged tetanic stimulation of single fibres from Xenopus skeletal muscle. J Physiol Lond 492: 723-736.
Zhao M, Hollingworth S and Baylor SM (1997). AM-loading of fluorescent Ca2+ indicators into intact single fibres of frog muscle. Biophys J 72: 2736-2747.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caputo, C., Bolaños, P. & Escobar, A.L. Fast calcium removal during single twitches in amphibian skeletal muscle fibres. J Muscle Res Cell Motil 20, 555–567 (1999). https://doi.org/10.1023/A:1005526202747
Issue Date:
DOI: https://doi.org/10.1023/A:1005526202747