Abstract
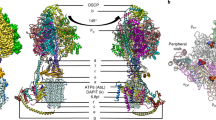
Membrane-bound ATP synthases (F1F0) catalyze the synthesis of ATP via a rotary catalyticmechanism utilizing the energy of an electrochemical ion gradient. The transmembrane potentialis supposed to propel rotation of a subunit c ring of F0 together with subunits γ and ∈ of F1,hereby forming the rotor part of the enzyme, whereas the remainder of the F1F0 complexfunctions as a stator for compensation of the torque generated during rotation. This reviewfocuses on our recent work on the stator part of the F0 complex, e.g., subunits a and b. Usingepitope insertion and antibody binding, subunit a was shown to comprise six transmembranehelixes with both the N- and C-terminus oriented toward the cytoplasm. By use of circulardichroism (CD) spectroscopy, the secondary structure of subunit b incorporated intoproteoliposomes was determined to be 80% α-helical together with 14% β turn conformation, providingflexibility to the second stalk. Reconstituted subunit b together with isolated ac subcomplexwas shown to be active in proton translocation and functional F1 binding revealing the nativeconformation of the polypeptide chain. Chemical crosslinking in everted membrane vesiclesled to the formation of subunit b homodimers around residues bQ37 to bL65, whereas bA32Ccould be crosslinked to subunit a, indicating a close proximity of subunits a and b near themembrane. Further evidence for the proposed direct interaction between subunits a and b wasobtained by purification of a stable ab 2 subcomplex via affinity chromatography using Histags fused to subunit a or b. This ab 2 subcomplex was shown to be active in proton translocationand F1 binding, when coreconstituted with subunit c. Consequences of crosslink formationand subunit interaction within the F1F0 complex are discussed.
Similar content being viewed by others
REFERENCES
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994). Nature (London) 370, 621–628.
Altendorf, K, Stalz, W.-D., Greie, J.-C., and Deckers-Hebestreit, G. (2000). J. Exp. Biol. 203, 19–28.
Aris, J. P. and Simoni, R. D. (1983). J. Biol. Chem. 258 , 14599–14609.
Bjørbæk, C., Foërsom, V., and Michelsen, O. (1990). FEBS Lett. 260,31–34.
Böttcher, B., Schwarz, L., and Gräber, P. (1998). J. Mol. Biol. 281, 757–762.
Böttcher, B., Bertsche, I., Reuter, R., and Gräber, P. (2000). J. Mol. Biol. 296,449–457.
Cherepanov, D. A., Mulkidjanian, A. Y, and Junge, W. (1999). FEBS Lett. 449, 1–6.
Deckers-Hebestreit, G. and Altendorf, K. (1992). J. Exp. Biol. 172,451–459.
Deckers-Hebestreit, G. and Altendorf, K (1996). Annu. Rev. Microbiol. 50,791–824.
Deckers-Hebestreit, G., Greie, J.-C., Stalz, W.-D., and Altendorf, K (2000). Biochim. Biophys. Acta 1458, 364–373.
Dmitriev, O. Y, Jones, P. C., Jiang, W., and Fillingame, R. H. (1999). J. Biol. Chem. 274, 15598–15604.
Dunn, S. D. (1992). J. Biol. Chem. 267, 7630–7636.
Dunn, S. D. and Chandler, J. (1998).J. Biol. Chem. 273, 8646–8651.
Dunn, S. D., McLachlin, D. T., and Revington, M. (2000). Biochim. Biophys. Acta 1458, 356–363.
Fillingame, R. H. (1990). In The Bacteria. A Treatise on Structure and Function, Vol. 12 (Krulwich, T. A., ed.), Academic Press, New York, pp. 345–391.
Fillingame, R. H., Jiang, W., Dmitriev, O. Y, and Jones, P. C. (2000). Biochim. Biophys. Acta 1458, 387–403.
Fraga, D. and Fillingame, R. H. (1991). J. Bacterial. 173 , 2639–2643.
Fraga, D., Hermolin, J., Oldenburg, M., Miller, M. J., and Fillingame, R. H. (1994). J. BioI. Chem. 269, 7532–7537.
Girvin, M. E., Rastogi, V. K, Abildgaard, F, Markley, J. L., and Fillingame, R. H. (1998). Biochemistry 37, 8817–8824.
Gogol, E. P., Lücken, U., and Capaldi, R. A. (1987). FEBS Lett. 219, 274–278.
Greie, J.-C., Deckers-Hebestreit, G., and Altendorf, K. (2000). Eur. J. Biochem. 267, 3040–3048.
Hermolin, J., Gallant, J., and Fillingame, R. H. (1983). J. BioI. Chem. 258, 14550–14555.
Hermolin, J., Dmitriev, O. Y, Zhang, Y, and Fillingame, R. H. (1999). J. BioI. Chem. 274, 17011–17016.
Jäger, H., Birkenhäger, R., Stalz, W.-D., Altendorf, K, and Deckers-Hebestreit, G. (1998). Eur. J. Biochem. 251, 122–132.
Jiang, W and Fillingame, R. H. (1998). Proc. Natl. Acad. Sci. USA 95, 6607–6612.
Jones, P. C., Hermolin, J., Jiang, W., and Fillingame, R. H. (2000). J. Biol. Chem. 275, 31340–31346.
Junge, W (1999). Proc. Natl. Acad. Sci. USA 96, 4735–4737.
Kumamoto, C. A. and Simoni, R. D. (1986). J. Biol. Chem. 261 , 10037–10042.
Lewis, M. J., Chang, J. A., and Simoni, R. D. (1990). J. Biol. Chem. 265, 10541–10550.
Lill, H., Hensel, F, Junge, W, and Engelbrecht, S. (1996). J. Biol. Chem. 271, 32737–32742.
Long, J. C., Wang, S., and Vik, S. B. (1998). J. Biol. Chem. 273 , 16235–16240.
McLachlin, D. T. and Dunn, S. D. (1997). J. Biol. Chem. 272 , 21233–21239.
McLachlin, D. T. and Dunn, S. D. (2000). Biochemistry 39 , 3486–3490.
McLachlin, D. T., Bestard, J. A., and Dunn, S. D. (1998). J. Biol. Chem. 273, 15162–15168.
McLachlin, D. T., Coveny, A. M., Clark, S. M., and Dunn, S. D. (2000). J. Biol. Chem. 275, 17571–17577.
Masaike, T., Mitome, N., Noji, H., Muneyuki, E., Yasuda, R., Kinosita, K., Jr., and Yoshida, M. (2000). J. Exp. Biol. 203, 1–8.
Meunier, B. and Rich, P. R. (1998). J.Mol. Biol. 283,727–730.
Miller, M. J., Fraga, D., Paule, C. R., and Fillingame, R. H. (1989). J. Biol. Chem. 264, 305–311.
Mosher, M. E., White, L. K, Hermolin, J., and Fillingame, R. H. (1985). J. Biol. Chem. 260, 4807–4814.
Nakamoto, R. K, Ketchum, C. J., and Al-Shawi, M. K (1999). Annu. Rev. Biophys. Biomol. Struct. 28, 205–234.
Ogilvie, I., Aggeler, R., and Capaldi, R. A. (1997). J. Biol. Chem. 272, 16652–16656.
Pänke, O., Gumbiowski, K., Junge, W., and Engelbrecht, S. (2000). FEBS Lett. 472, 34–38.
Patterson, A. R., Wada, T., and Vik, S. B. (1999). Arch. Biochem. Biophys. 368, 193–197.
Rastogi, V. K. and Girvin, M. E. (1999). Nature (London) 402 , 263–268.
Rodgers, A. J. W and Capaldi, R. A. (1998). J. Biol. Chem. 273 , 29406–29410.
Rodgers, A. J. W., Wilkens, S., Aggeler, R., Morris, M. B., Howitt, S. M., and Capaldi, R. A. (1997). J. Biol. Chem. 272 , 31058–31064.
Sambongi, Y, Iko, Y, Tanabe, M., Omote, H., Iwamoto-Kihara, A., Ueda, I., Yanagida, T., Wada, Y, and Futai, M. (1999). Science 286, 1722–1724.
Schneider, E. and Altendorf, K (1984). Proc. Natl. Acad. Sci. USA 81,7279–7283.
Schneider, E. and Altendorf, K. (1985). EMBO J. 4, 515–518.
Schulenberg, B., Wellmer, F, Lill, H., Junge, W., and Engelbrecht, S. (1997). Eur. J. Biochem. 249, 134–14l.
Schulenberg, B., Aggeler, R., Murray, J., and Capaldi, R. A. (1999). J. Biol. Chem. 274, 34233–34237.
Senior, A. E. (1983). Biochim. Biophys. Acta 726, 81–95.
Sorgen, P. L., Bubb, M. R., McCormick, K A., Edison, A. S., and Cain, B. D. (1998a) Biochemistry 37, 923–932.
Sorgen, P. L., Caviston, T. L., Perry, R. C., and Cain, B. D. (1998b). J. Biol. Chem. 273, 27873–27878.
Steffens, K, Schneider, E., Deckers-Hebestreit, G., and Altendorf, K (1987). J. Biol. Chem. 262, 5866–5869.
Stock, D., Leslie, A. G. W., and Walker, J. E. (1999). Science 286, 1700–1705.
Takeyama, M., Noumi, T., Maeda, M., and Futai, M. (1988). J. Biol. Chem. 263, 16106–16112.
Tang, C. and Capaldi, R. A. (1996). J. Biol. Chem. 271, 3018–3024.
Uhlin, U., Cox, G. B., and Guss, J. M. (1997). Structure 5 , 1219–1230.
Valiyaveetil, F. I. and Fillingame, R. H. (1998). J. Biol. Chem. 273, 16241–16247.
Wada, T., Long, J. C., Zhang, D., and Vik, S. B. (1999). J. Biol. Chem. 274, 17353–17357.
Walker, J. E., Saraste, M., and Gay, N. J. (1982). Nature (London) 298, 867–869.
Wang, H. and Oster, G. (1998). Nature (London) 396, 279–282.
Watts, S. D., Tang, C., and Capaldi, R. A. (1996). J. Biol. Chem. 271, 28341–28347.
Wilkens, S. and Capaldi, R. A. (1998). Nature (London) 393, 29.
Wilkens, S., Dahlquist, F W., McIntosh, L. P., Donaldson, L. W., and Capaldi, R. A. (1995). Natur. Struct. Biol. 2, 961–967.
Wilkens, S., Dunn, S. D., Chandler, J., Dahlquist, E W., and Capaldi, R. A. (1997). Natur. Struct. Biol. 4, 198–20l.
Wilkens, S., Zhou, J., Nakayama, R., Dunn, S. D., and Capaldi, R. A. (2000). J. Mol. Biol. 295, 387–39l.
Yamada, H., Moriyama, Y., Maeda, M., and Futai, M. (1996). FEBS Lett. 390, 34–38.
Yasuda, R., Noji, H., Kinosita, K, Jr., and Yoshida, M. (1998). Cell 93, 1117–1124.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Greie, JC., Deckers-Hebestreit, G. & Altendorf, K. Subunit Organization of the Stator Part of the F 0 Complex from Escherichia coli ATP Synthase . J Bioenerg Biomembr 32, 357–364 (2000). https://doi.org/10.1023/A:1005523902800
Issue Date:
DOI: https://doi.org/10.1023/A:1005523902800