Abstract
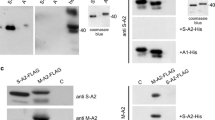
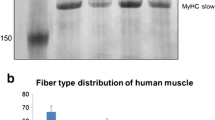
In higher vertebrates, the glycolytic enzyme enolase (2-phospho-d- glycerate hydrolyase; EC 4.2.1.11) is active as a dimeric protein formed from three subunits – α: ubiquitous, β: muscle specific, and γ: neuron specific – encoded by different genes. In the present study, we have shown that an antiserum previously produced against the mouse ββ enolase is also a specific reagent for the muscle specific human enolase. Using this antiserum to study human muscles, we demonstrated novel patterns of the β subunit microheterogeneity which are distinctive from those observed previously in rodents and which appear to be independent of age, gender and muscular activity. Two variants of the beta subunit differing by their size have been detected: one heavy form of 46 kDa (βH) and one light form of 45 kDa (βL). Muscle biopsies expressed either βH or βL or βH + βL, and all muscles of an individual expressed the same variants. The products of in vitro translation of RNA prepared from human muscle displayed β subunit variants identical to those of the protein present in the biopsy. Therefore the differences observed between individuals reveal a difference already present at the level of the RNA transcripts. These observations suggest the existence of an yet undescribed polymorphism of the human β enolase gene which could affect the coding sequence. Comparative immunocytochemical and histochemical analyses of biopsies demonstrated that the β subunit was expressed in all fast fibres (type II), but not in slow fibres (type I). No difference was observed in the intensity of β enolase immunolabelling between the various types (IIA, IIAB, IIB) of fast fibres. No significant difference in fibre type composition and histological appearance was visible between muscles presenting either one of the three patterns of microheterogeneity.
Similar content being viewed by others
References
ARRIO-DUPONT, M., FOUCAULT, G., VACHER, M., DOUHOU, A. & CRIBIER, S. (1997) Mobility of creatine phosphokinase and beta-enolase in cultured muscle cells. Biophys J. 73, 2667-2673.
BARBIERI, G., DE ANGELIS, L., FEO, S., COSSU, G. & GI-ALLONGO, A. (1990) Differential expression of muscle-specific enolase in embryonic and fetal myogenic cells during mouse development. Differentiation 45, 179-184.
BRADFORD, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254.
CRAIG, S. P., DAY, I. N., THOMPSON, R. J. & CRAIG, I. W. (1990) Localisation of neurone-specific enolase (ENO2) to 12p13. Cytogenet Cell Genet. 54, 71-73.
DUBOWITZ, V. (1985). Muscle biopsy: A practical approach. 2nd ed. London: BallieÁre Tindall.
FEO, S., OLIVA, D., BARBIERI, G., XU, W. M., FRIED, M. & GIALLONGO, A. (1990) The gene for the muscle-specific enolase is on the short arm of human chromosome 17. Genomics 6, 192-194.
FLETCHER, L., RIDER, C., TAYLOR, C., ADAMSON, E., LUKE, B. & GRAHAM, C. (1978) Enolase isoenzymes as markers of differentiation in teratocarcinoma cells and normal tissues of mouse. Dev Biol. 65, 462-475.
GIALLONGO, A., VENTURELLA, S., OLIVA, D., BARBIERI, G., RUBINO, P. & FEO, S. (1993) Structural features of the human gene for muscle-specific enolase. Differential splicing in the 5'-untranslated sequence generates two forms of mRNA [published erratum appears in Eur J Biochem 1993 Dec 15;218(3):1095]. Eur J Biochem. 214, 367-374.
HUGHES, S. M., CHO, M., KARSCH, M. I., TRAVIS, M., SI-LBERSTEIN, L., LEINWAND, L. A. & BLAU, H. M. (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol. 158, 183-199.
IBI, T., SABASHI, K., KATO, K., TAKAHASHI, A. & SOBUE, I. (1983) Immuno histochemical demonstration of b-enolase in human skeletal muscle. Muscle and Nerve 6, 661-663.
KADI, F., HAGG, G., HAKANSSON, R., HOLMNER, S., BUTLERBROWNE, G. S. & THORNELL, L. E. (1998) Structural changes in male trapezius muscle with workrelated myalgia. Acta Neuropathol. 95, 352-360.
KATO, K., SHIMIZU, A., SEMBA, R. & SATOH, T. (1985) Tissue distribution, developmental profiles and effect of denervation of enolase isozymes in rat muscles. Biochim Biophys Acta. 841, 50-58.
KELLER, A., BEROD, A., DUSSAILLANT, M., LAMANDE, N., GROS, F. & LUCAS, M. (1994) Coexpression of alpha and gamma enolase genes in neurons of adult rat brain. J Neurosci Res. 38, 493-504.
KELLER, A., OTT, M. O., LAMANDE, N., LUCAS, M., GROS, F., BUCKINGHAM, M. & LAZAR, M. (1992) Activation of the gene encoding the glycolytic enzyme beta-enolase during early myogenesis precedes an increased expression during fetal muscle development. Mech Dev. 38, 41-54.
KELLER, A., ROUZEAU, J. D., FARHADIAN, F., WISNEWSKY, C., MAROTTE, F., LAMANDE, N., SAMUEL, J. L., SCHWARTZ, K., LAZAR, M. & LUCAS, M. (1995) Differential expression of alpha and beta-enolase genes during rat heart development and hypertrophy. Amer J Physiol-Heart Circ Phy. 38, H1843-H1851.
KELLER, A., SCARNA, H., MERMET, T. & PUJOL, J. F. (1981) Biochemical and immunological properties of the mouse brain enolases purified by a simple method. J. Neurochem. 36, 1389-1397.
KORGE, P. & CAMPBELL, K. (1995) The importance of ATPase microenvironment in muscle fatigue: a hypothesis. Int. J. Sports Med. 16, 172-179.
LUCAS, M., GOBLET, C., KELLER, A., LAMANDE, N., GROS, F., WHALEN, R. G. & LAZAR, M. (1992) Modulation of embryonic and muscle-specific enolase gene products in the developing mouse hindlimb. Differentiation 51, 1-7.
LUTHER, M. A. & LEE, J. C. (1986) The role of phosphorylation in the interaction of rabbit muscle phosphofructokinase with F-actin. J. Biol. Chem. 261, 1753-1759.
MASTERS, C. (1992) Microenvironmental factors and the binding of glycolytic enzymes to contractile filaments. Int J Biochem. 24, 405-410.
MERKULOVA, T., LUCAS, M., JABET, C., LAMANDE, N., ROUZEAU, J. D., GROS, F., LAZAR, M. & KELLER, A. (1997) Biochemical characterization of the mouse muscle-specific enolase: Developmental changes in electrophoretic variants and selective binding to other proteins. Biochem J. 323, 791-800.
MILES, L. A., DAHLBERG, C. M., PLESCIA, J., FELEZ, J., KATO, K. &PLOW, E. F. (1991) Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 30, 1682-1691.
MORISSEY, M. (1981) Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117, 307-310.
POLLARD, K., CHAN, E., GRANT, B., SULLIVAN, K., TAN, E. & GLASS, C. (1990) In vitro posttranslational modification of lamin B cloned from a human T-cell line. Mol Cell Biol. 10, 2164-2175.
REDLITZ, A., FOWLER, B. J., PLOW, E. F. & MILES, L. A. (1995) The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 227, 407-415.
STERNBERGER, L. A. (1979). Immunocytochemistry. New York: Wiley.
ZHAO, S. M. & LEE, E. Y. C. (1997) Targeting of the catalytic subunit of protein phosphatase-1 to the glycolytic enzyme phosphofructokinase. Biochemistry 36, 8318-8324.
ZOMZELY-NEURATH, C. (1983) Enolase. Enzymes in the nervous system, In Handbook of Neurochemistry (edited by LAJTHA, A. ) New York: Plenum.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Merkulova, T., Thornell, LE., Butler-Browne, G. et al. The β enolase subunit displays three different patterns of microheterogeneity in human striated muscle. J Muscle Res Cell Motil 20, 55–63 (1999). https://doi.org/10.1023/A:1005428328913
Issue Date:
DOI: https://doi.org/10.1023/A:1005428328913