Abstract
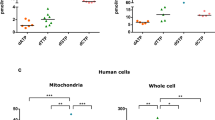
We performed comparative biochemical studies in cultured fibroblast mitochondria from patients with the T8993G or the T8993C point mutations in the ATPase 6 gene of mitochondrial DNA. We found that ATP production was much more severely decreased in cells from patients with the T8993G mutation than in those from patients with the T8993C mutation. Kinetic studies suggest that both mutations affect only the F0 sector of the mitochondrial ATPase complex. We conclude that these two mutations, which result in the substitution of different amino acids at the same site of the ATPase, result in an enzyme with different biochemical characteristics.
Similar content being viewed by others
REFERENCES
Abrahams JP, Leslie AGW, Lutter R, Walker JE (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621-628.
Amzel LM, Bianchet MA, Pedersen PL (1992) Quaternary structure of ATP synthases: symmetry and asymmetry in the F1 moiety. J Bioenerg Biomembr 24: 429-433.
Anderson S, Bankier AT, Barrel BG, et al (1981) Sequence and organization of the human mitochondrial genome. Nature 290: 457-465.
Boyer PD (1993) The binding change mechanism for ATP synthase-some probabilities and possibilities. Biochim Biophys Acta 1140: 215-250.
Cain BD, Simoni RD (1988) Interaction between Glu-219 and His-245 within the a subunit of in Escherichia coli. J Biol Chem 263: 6606-6612. F1F0-ATPase
Cain BD, Simoni RD (1989) Proton translocation by the ATPase of E. coli: mutagenic F1F0 analysis of the a subunit. J Biol Chem 264: 3292-3300.
Calanni RF, Baracca A, Solaini G et al (1986) Effects of cholesterol on the kinetics of mitochondrial ATPase. FEBS Lett 198: 353-356.
deVries DD, van Engelen BGM, Gabreels FJM, et al. (1993) A second missense mutation in the mitochondrial ATPase6 gene in LeighÏ s syndrome. Ann Neurol 34: 410-412.
Estabrook RW (1967) Mitochondrial respiratory control and the polarographic measurement of ADP: O ratios. In Eastabrook RW, Pullman M, eds. Methods in Enzymology vol. 10. Academic Press, London, 41.
Fillingame RH (1980) The proton translocating pumps of oxidative phosphorylation. Annu Rev Biochem 49: 1079-1113.
Fillingame RH (1992) H+ transport and coupling by the sector of the ATP synthase: F0 insights into the molecular mechanisms of function. J Bioenerg Biomembr 24: 485-491.
Glaser E, Norling B, Kopecky J, Ernster L (1982) Comparison of the effects of oligomycin and dicyclohexylcarbodiimide on mitochondrial ATPase and related reactions. Eur J Biochem 121: 525-531.
Hatefi Y (1993) ATP synthesis in mitochondria. Eur J Biochem 218: 759-767.
Holme E, Greter J, Jacobson CE, et al (1992) Mitochondrial ATP-synthase deficiency in a child with 3-methylglutaconic aciduria. Pediatr Res 32: 731-735.
Holt IJ, Harding AE, Petty RKH, Morgan-Hughes, JA (1990) A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 46: 428-433.
Houstek J, Klement P, Hermanska J, et al (1995) Altered properties of mitochondrial ATP synthase in patients with a T]G mutation in the ATPase 6 (subunit a) gene at position 8993 of mtDNA. Biochim Biophys Acta 1271: 349-357.
Lee CP, Azzone GF, Ernster L (1964) Evidence for energy coupling in nonphosphorylating electron transport particles from beef heart mitochondria. Nature 201: 152-155.
Millis AJT, Pious DA (1973) Oxidative phosphorylation in mitochondria isolated from human fibroblast. Biochim Biophys Acta 292: 73-77.
Penefsky HS (1985) Mechanism of inhibition of mitochondrial adenosine triphosphatase by dicyclohexylcarbodiimide and oligomycin: relationship to ATP synthesis. Proc Natl Acad Sci USA 82: 1589-1593.
Pullman ME, Penefsky HS, Datta A, Racker E (1960) Partial resolution of the enzymes catalyzing oxidative phosphorylation. I: Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem 235: 3322-3329.
Santorelli FM, Shanske S, Jain KD, et al (1994) A T→C mutation at nt 8993 of mitochondrial DNA in a child with Leigh syndrome. Neurology 44: 972-974.
Summer JB (1944) A method for the colorimetric determination of phosphorus. Science 100: 413-414.
Tatuch Y, Robinson BH (1993) The mitochondrial DNA mutation at 8993 associated with NARP slows the rate of ATP synthesis in isolated lymphoblast mitochondria. Biochem Biophys Res Commun 192: 124-128.
Tatuch Y, Christodoulou J, Feigenbaum A et al (1992) Heteroplasmic mtDNA mutation (T→G) can cause Leigh disease when the percentage of abnormal mtDNA is high. Am J Hum Genet 50: 852-858.
Tuena de Gómez-Puyou M, Ayala G, Darszon A, Gómez-Puyou A (1984) Oxidative phosphorylation and the Pi-ATP exchange reaction of submitochondrial particles under the influence of organic solvents. J Biol Chem 259: 9472-9478.
Vázquez-Memije ME, Shanske S, Santorelli FM et al (1996) Comparative biochemical studies in fibroblasts from patients with different forms of Leigh syndrome. J Inher Metab Dis 19: 43-50.
Walker JE, Collinson IR (1994) The role of the stalk in the coupling mechanism of F1F0-ATPases. FEBS Lett 346: 39-43.
Zanotti F, Guerrieri F, Capozzo G et al (1992) Role of and subunits in the gating and F0 F1 coupling function of mitochondrial H+ ATP synthase: the effect of dithiol reagents. Eur J Biochem 208: 9-16.
Rights and permissions
About this article
Cite this article
Vázquez-Memije, M.E., Shanske, S., Santorelli, F.M. et al. Comparative biochemical studies of ATPases in cells from patients with the T8993G or T8993C mitochondrial DNA mutations. J Inherit Metab Dis 21, 829–836 (1998). https://doi.org/10.1023/A:1005418718299
Issue Date:
DOI: https://doi.org/10.1023/A:1005418718299