Abstract
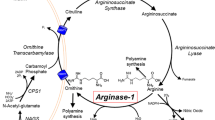
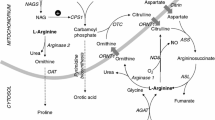
Arginase is the final enzyme in the urea cycle. Its deficiency is the least frequently described disorder of this cycle. It results primarily in elevated blood arginine, and less frequently in either persistent or acute elevations in blood ammonia. This appears to be due to a second arginase locus, expressed primarily in the kidney, which can be recruited to compensate, in part, for the deficiency of liver arginase. The liver arginase gene structure permitted study of the molecular pathology of patients with the disorder and the results of these studies and the inferences about the protein structure are presented. The conserved regions among all arginases allowed the cloning of AII, the second arginase isoform. It has been localized to the mitochondrion and is thought to be involved in ornithine biosynthesis. It shares the major conserved protein sequences, and structural features of liver arginase gene are also conserved. When AI and AII from various species are compared, it appears that the two diverged some time prior to the evolution of amphibians. The evidence for the role of AII in nitric oxide and polyamine metabolism is presented and this appears consonant with the data on the tissue distribution.
Similar content being viewed by others
REFERENCES
Ash DE, Scolnick LR, Kanyo ZF, Vockley JG, Cederbaum SD, Christianson DW (1998) Molecular basis of argininemia: structure-function consequences of mutations in human liver arginase. Mol Genet Metab, in press.
Brusilow SW, Horwich AL (1995) Urea cycle enzymes. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Diseases, 7th edn. New York: McGraw-Hill, 621–663.
Buga GM, Singh R, Pervin S, Jenkinson CP, Cederbaum SD, Ignarro LJ (1996) Arginase activity in endothelial cell: inhibition by NG-hydroxyarginine during high-output nitric oxide production. Am J Physiol 271: H1988–H1998.
Cederbaum SD, Moedjono SJ, Shaw KNF, Naylor EW, Walser M, Carter M (1982) Treatment of hyperargininaemia due to arginase deficiency. J Inter Metab Dis 5: 95–99.
Cederbaum SD, Shaw KNF, Spector EB, Verity MA, Snodgrass PJ, Sugarman GI (1979) Hyperargininemia due to arginase deficiency. Pediatr Res 13: 827–833.
Cerone R, Coruso U, Barabino A et al (1997) Hyperargininemia: pre-natal diagnosis and treatment from birth. In De Deyn PP, Marescau B, Qureshi IA, Mori A, eds. Guanidino Compounds in Biology and Medicine II. London: John Libbey, 71–75.
De Deyn PP, Marescau B, Qureshi IA et al (1997) Hyperargininemia: a treatable inborn error of metabolism. In De Deyn PP, Marescau B, Qureshi IA, Mori A. eds. Guanidino Compounds in Biology and Medicine II. London: John Libbey, 53–69.
Dickinson JC, Hamilton PB (1966) The free amino acids of human spinal fluid determined by ion exchange chromatography. J Neurochem 13: 1179–1187.
Dizikes GJ, Grody WW, Kern RM, Cederbaum SD (1986a) Isolation of human arginase cDNA and absence of homology between the two human arginase genes. Biochem Biophys Res Commun 141: 53–59.
Dizikes GJ, Spector EB, Cederbaum SD (1986b) Cloning of rat liver arginase cDNA and the elucidation of the regulation of arginase gene expression in H4 rat hepatoma cells. Somat Cell Mol Genet 12: 375–384.
Glass RD, Knox WE (1973) Arginase isozymes of rat mammary gland, liver and other tissues. J Biol Chem 248: 5785–5789.
Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M (1996) Molecular cloning of cDNA for non-hepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395: 119–122.
Gotoh T, Araki M, Mori M (1997) Chromosomal localization of the human arginase II gene and tissue distribution of its mRNA. Biochem Biophys Res Commun 233: 487–491.
Grody WW, Argyle C, Kern RM, et al (1989) Differential expression of the two human arginase genes in hyperargininemia: enzymatic pathologic and molecular analysis J Clin Invest 83: 602–609.
Grody WW, Kern RM, Klein D, et al (1993) Arginase deficiency manifesting delayed clinical sequelae and induction of a kidney arginase isozyme. Hum Genet 91: 1–5.
Haraguchi Y, Takiguchi M, Amaya Y, Kawamoto S, Matsuda I, Mori M (1987) Molecular cloning and nucleotide sequence of cDNA for human liver arginase. Proc Natl Acad Sci USA 84: 412–415.
Jenkinson CP, Grigor MR (1994) Rat mammary gland arginase: isolation and characterization. Biochem Med Metab Biol 51: 156–165.
Jenkinson CP, Grody WW, Cederbaum SD (1996) Comparative properties of arginases. Comp Biochem Physiol 114B: 107–132.
Jenkinson CP, Iyer RK, Rao N, et al (1997) Isolation and analysis of human arginase II genomic clones. Am J Hum Genet (Abstract) 61: A355.
Kanyo ZF, Scolnick LR, Ash DE, Christianson DW (1996) Structure of a unique binuclear manganese cluster in arginase. Nature 383: 554–557.
Kaysen GA, Strecker HJ (1973) Purification and properties of arginase of rat kidney. Biochem J 133: 779–788.
Kidd JR, Dizikes GJ, Grody WW, Cederbaum SD, Kidd KK (1986) A PvuII RFLP for the human liver arginase (ARG1) gene. Nucleic Acids Res 14: 9544.
Kim PS, Iyer RK, Kern RM, et al (1997) Cloning and sequencing of mouse and rat arginase AII genes. Am J Hum Genet (Abstract) 61: A176.
Kocna P, Fric P, Zavoral M, Pelech T (1996) Arginase activity determination. A marker of large bowel mucosal proliferation. Eur J Clin Biochem 34: 619–623.
Marescau B, DeDeyn PP, Quresh IA, et al (1992) The pathobiochemistry of uremia and hyperargininemia further demonstrates a metabolic relationship between urea and guanidinosuccinic acid. Metabolism 41: 1021–1024.
Mori M, Gotoh T, Nagasaki A, Takiguchi M, Sonoki T (1998) Regulation of the urea cycle enzyme genes in nitric oxide synthesis. J. Inher Metab Dis 21 Suppl 1: 59–71.
Morris SM Jr, Bhamidipati D, Kepta-Lenhart D (1997) Human type II arginase: sequence analysis and tissue-specific expression. Gene 193: 157–161.
Patterson D, Shi YB (1994) Thyroid hormone-dependent differential regulation of multiple arginase genes during amphibian metamorphosis. J Biol Chem 269: 25328–25334.
Qureshi IA, Letarte J, Ouellet R, Lelievre M (1981) Sodium benzoate therapy and dietary control in hyperargininemia. Pediatr Res 15: 6–38.
Peralta-Serrano A (1965) Argininuria, convulsiones y oligofrenia. Un nuevo error innata del metabolismo? Rev Clin Esp 97: 176–184.
Snyderman SE, Sansaricq C, Norton PM, Goldstein FJ (1979) Argininemia treated from birth. J. Pediatr 95: 61–63.
Sonoki T, Nagasaki A, Gotoh T, et al (1997) Coinduction of nitric oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysuccharide. J Biol Chem 272: 3689–3693.
Sparkes RS, Dizikes GJ, Klisak I, et al (1986) The gene for human liver arginase (AI) is assigned to chromosome band 6q23. Am J Hum Genet 39: 186–193.
Spector EB, Kiernan M, Bernard B, Cederbaum SD (1980) Comparative properties of fetal and adult red blood cell arginase: a possible prenatal diagnostic test for arginase deficiency. Am J Hum Genet 32: 79–87.
Spector EB, Rice SCH, Moedjono S, Bernard B, Cederbaum SD (1982) Biochemical properties of arginase in human adult and fetal tissues. Biochem Med 28: 165–175.
Spector EB, Rice SCH, Cederbaum, SD (1983) Immunologic studies of arginase in tissues of normal human adults and arginase-deficient patients. Pediatr Res 17: 941–944.
Spector EB, Jenkinson CP, Grigor MR, Kern RM, Cederbaum SD (1994) Subcellular location and differential antibody specificity of arginase in tissue culture and whole animals. Int J Dev Neurosci 12: 337–342.
Takiguchi M, Haraguchi Y, Mori M (1988) Human liver-type arginase gene: structure and gene analysis of the promotor region. Nucleic Acids Res 16: 8789–8802.
Terheggen HF, Schwenk A, Lowenthal A, van Sande M, Colombo JP (1970a) Hyperargininemia with arginase deficiency, a new familial metabolic disease: clinical aspects. Z Kinderheilk 107: 298–312.
Terheggen HF, Schwenk A, Lowenthal A, van Sande M, Colombo JP (1970b) Hyperargininemia with arginase deficiency, a new familial metabolic disease: biochemical aspects. Z Kinderheilk 107: 313–323.
Uchino T, Snyderman SE, Lambert M, et al (1995) Molecular basis of phenotype variation in patients with argininemia. Hum. Genet 96: 255–260.
Vockley JG, Goodman BK, Tabor DE, et al (1996a) Loss-of-function mutations in conserved regions of the human liver arginase gene. Biochem Mol Med 59: 44–51.
Vockley JG, Jenkinson CP, Shuklal H, Kern RM, Grody WW, Cederbaum SD (1996b) Cloning and characterization of the human type II arginase gene. Genomics 38: 118–123.
Wang WW, Jenkinson CP, Griscavage JM, et al (1995) Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochem Biophys Res Commun 210: 1009–1016.
Rights and permissions
About this article
Cite this article
Iyer, R., Jenkinson, C.P., Vockley, J.G. et al. The human arginases and arginase deficiency. J Inherit Metab Dis 21 (Suppl 1), 86–100 (1998). https://doi.org/10.1023/A:1005313809037
Issue Date:
DOI: https://doi.org/10.1023/A:1005313809037