Abstract
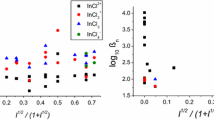
The association constant for the reaction: \({\text{Mg}}^{{\text{2 + }}} + {\text{SO}}_{\text{4}}^{{\text{2 - }}} \rightleftharpoons {\text{MgSO}}_{\text{4}}\) has been measured at 25°C using magnesium ion-selective electrode (Mg–ISE) potentiometry in aqueous solutions of ionic strength (I) ranging from 0.25 to 6 M in CsCl and in 1 M (Me4NCl). The value of log β(MgSO4) = 0.98 ± 0.02 in 1 M (Me4NCl) was significantly higher than that of 0.75 ± 0.01 obtained in 1 M(CsCl). This difference can be explained by a weak association between Cs+and SO 2-4 , with log β(CsSO -4 ) = −0.11 ± 0.03, which is also qualitatively consistent with the absence of an increase in β(MgSO4) at high ionic strength in CsCl media. Extrapolation of the results in CsCl media gave an infinite dilution value of log β° (MgSO4) = 2.38 ± 0.03 that was rather dependent on the nature of the extrapolation function. The performance of the Mg–ISE in various media is also briefly described.
Similar content being viewed by others
REFERENCES
B. I. Whittington and D. M. Muir, Processes Extr. Metallurg. Rev, 21, 527 (2000).
J. P. Riley and G. Skirrow, Chemical Oceanography (Academic Press, London, 1975).
M. Eigen and K. Tamm, Z. Elektrochem. 66, 107 (1962).
S. Katayama, Bull. Chem. Soc. Jpn. 46, 106 (1973).
R. M. Izatt, D. Eatough, J. J. Christensen, and C. H. Bartholomew, J. Chem. Soc. A 45, 47 (1969).
J. G. Norby, Acta Chem. Scand. 24, 3276 (1970).
F. P. Daly, C. W. Brown, and D. R. Kester, J. Phys. Chem. 76, 3664 (1972).
D. R. Kester and R. M. Pytkowicz, Limnol. Oceanogr. 13, 670 (1968).
B. Elgquist and M. Wedborg, Marine Chem. 3, 215 (1975); Marine Chem. 6, 243 (1978).
L. G. Sillen and A. E. Martell, Stability Constants of Metal-Ion Complexes, Spec. Publ. 17 and 25 (The Chemical Society, London, 1964, 1971).
K. S. Pitzer, Activity Coefficients in Electrolyte Solutions (CRC Press, Boca Raton, Florida, 1991).
R. A. Robinson and R. H. Stokes, Electrolyte Solutions, 2nd edn. (Butterworths, London, 1970).
B. Elgquist and M. Wedborg, Marine Chem. 2, 1 (1974).
P. Verhoeven, G. T. Hefter, and P. M. May, Mineral Metal. Proc. 5, 185 (1990).
P. Sipos, I. Bodi, G. T. Hefter, and P. M. May, Talanta, 44, 617 (1997).
P. M. May, K. Murray, and D. R. Williams, Talanta 32, 483 (1985).
G. T. Hefter and A. R. Longmore, Inter. J. Environ. Anal. Chem. 16, 315 (1984).
G. T. Hefter, J. Solution Chem. 11, 45 (1982).
P. M. May, K. Murray, and D. R. Williams, Talanta 35, 825 (1988).
S. G. Capewell, G. Hefter, and P. M. May, J. Solution Chem. 27, 865 (1998).
G. H. Nancollas, Interactions in Electrolyte Solutions (Elsevier, New York, 1966).
S. G. Capewell, G. Hefter, P. Sipos, and P. M. May, J. Solution Chem. 26, 957 (1997).
S. G. Capewell, R. Buchner, G. Hefter, and P. M. May, Phys. Chem. Chem. Phys. 1, 1933 (1999).
S. G. Capewell, R. Buchner, G. Hefter, and P. M. May, J. Phys. Chem. B 103, 1185 (1999).
F. Malatesta and R. Zamboni, J. Solution Chem. 26, 791 (1997).
I. Grenthe, Modeling in Aquatic Chemistry (OECD Publications, Paris, 1997).
H. W. Jones and C. B. Monk, Trans. Faraday Soc. 48, 929 (1952).
S. G. Capewell, G. Hefter, and P. M. May, Talanta 49, 25 (1999).
Rights and permissions
About this article
Cite this article
Kratsis, S., Hefter, G. & May, P.M. Potentiometric Study of the Association of Magnesium and Sulfate Ions at 25°C in High Ionic Strength Media. Journal of Solution Chemistry 30, 19–29 (2001). https://doi.org/10.1023/A:1005201825524
Issue Date:
DOI: https://doi.org/10.1023/A:1005201825524