Abstract
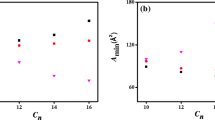
The zwitterionic micelles formed with two carboxybetaines and fivesulfobetaines have been investigated by micellar catalysis and dye solubility studieswith the probe Ni2+/PADA complexation reaction and the dye,pyridine-2-azo-p-dimethylaniline (PADA). Catalysis occurs with the carboxybetaines, butinhibition of the probe reaction arises with all of the sulfobetaines. Bothreactants enter carboxybetaine micelles, but Ni2+ ions are not appreciablyadsorbed by sulfobetaine micelles.
Similar content being viewed by others
REFERENCES
A. D. James and B. H. Robinson, J. Chem. Soc., Faraday Trans. I 74, 10 (1978).
C. D. Drennan, R. J. Hughes, V. C. Reinsborough, and O. O. Soriyan, Can. J. Chem, 76, 152 (1998).
E. A. G. Aniansson, S. N. Wall, M. Almgren, H. Hoffmann, I. Kielmann, W. Ulbricht, R. Zana, J. Lang, and C. Tondre, J. Phys. Chem. 80, 905 (1976).
Y. Chevalier, Y. Storet, S. Pourchet, and P. Le Perche, Langmuir 7, 848 (1991).
E. G. Lomox, Amphoteric Surfactants, Surfactant Science Series, Vol. 59, 2nd edn. (Marcel Dekker, New York, 1996), p. 4.
X. Domingo, Amphoteric Surfactants, Surfactant Science Series, Vol. 59, 2nd edn. (Marcel Dekker, New York, 1996), p. 75ff.
J. G. Weers, J. F. Rathman, F. U. Axe, C. A. Crichlow, L. D. Foland, D. R. Scheuing, R. J. Wiersema, and A. G. Zielske, Langmuir 7, 854 (1991).
T. J. Connolly, V. C. Reinsborough, and X. Xiang, Aust. J. Chem. 45, 769 (1992).
V. C. Reinsborough and B. H. Robinson, J. Chem. Soc., Faraday Trans. I 75, 2395 (1979).
Y. Moroi and K. Matsuoka, Bull. Chem. Soc. Jpn. 67, 2057 (1994).
D. G. Hall, J. Chem. Soc. Faraday Trans. II 77, 1973 (1981).
D. J. Jobe, R. E. Verrall, B. Skalski, and E. Aicart, J. Phys. Chem. 96, 6811 (1992).
P. D. Profio, R. Germani, G. Savelli, G. Cerichelli, M. Chiarini, G. Mancini, C. A. Bunton, and N. D. Gillitt, Langmuir 14, 2662 (1998).
K. K. Ghosh, A. Pandey, and S. Roy, Coll. Surf. A. 163, 293 (2000).
P. D. I. Fletcher, J. R. Hicks, and V. C. Reinsborough, Can. J. Chem. 61, 1594 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berberich, K.A., Reinsborough, V.C. & Shaw, C.N. Kinetic and Solubility Studies in Zwitterionic Surfactant Solutions. Journal of Solution Chemistry 29, 1017–1026 (2000). https://doi.org/10.1023/A:1005194903146
Issue Date:
DOI: https://doi.org/10.1023/A:1005194903146