Abstract
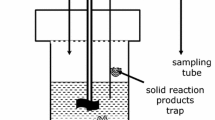
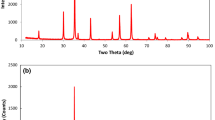
The solubility of Na2SO4 (s) (thenardite) and the interactions between magnetiteand aqueous Na2SO4 near the critical point of water have been determined in azirconium-alloy flow reactor at temperatures 350°C ≤ t ≤ 375°C and isobaricpressures 190 ≤ p ≤ 305 bar. The experimental solubility data are describedwell as a function of temperature and solvent density ρ1 byln x(Na2SO4, aq.) = −10.47 − 27550/T +(4805/T) ln ρ1.The interaction between magnetite and Na2SO4 (aq.) was examined from 250 to370°C at molalities near the saturation composition of Na2SO4 (s). While no solidreaction products were observed, HS− (aq.) was observed to form above 350°Cby sulfate reduction, as a product of the reaction8 Fe3O4(s) + Na2SO4 (aq.) + H2O(l)= 12 Fe2O3 (s) + NaHS (aq.) + NaOH (aq.).The reduction reaction appears to be controlled by surface reaction kinetics, ata level well below the equilibrium molality of HS− (aq.). Metallic iron reactedwith Na2SO4 (aq.) in a similar fashion at temperatures above 350°C, to yieldhigher molalities of HS− (aq.).
Similar content being viewed by others
REFERENCES
J. MacLairin, J. Chem. Soc. 63, 729 (1893)
J. P. Wuite, Z. Phys. Chem. 86, 349 (1913-1914).
W. C. Schroeder, A. Gabriel, and E. P. Partridge, J. Amer. Chem. Soc. 57, 1539 (1935).
N. B. Keevil, J. Amer. Chem. Soc. 64, 841 (1942).
H. S. Booth and R. M. Bidwell, J. Amer. Chem. Soc. 72, 2567 (1950).
W. L. Marshall, H. W. Wright, and C. H. Secoy, J. Chem. Educat. 72, 2567 (1950).
I. Kh. Khaibullin and B. E. Novikov, Teplofiz. Vys. Temp. 11, 320 (1973).
M. Obsil, V. Majer, G. T. Hefter, and V. Hynek, J. Chem. Eng. Data 42, 137 (1997).
M. Obsil, V. Majer, J.-P. E. Grolier, and G. T. Hefter, J. Chem. Soc. Faraday Trans. 92, 4445 (1996).
R. S. Z. Rogers and K. S. Pitzer, J. Phys. Chem. 85, 2886 (1981).
R. T. Pabalan and K. S. Pitzer, (a) Geochim. Cosmochim. Acta 51, 2429 (1987); (b) Geochim. Cosmochim. Acta 52, 2393 (1988).
K. S. Pitzer and J. S. Murdzek, J. Solution Chem. 11, 409 (1982).
M. L. Ravich and F. E. Borovaya, Russ. J. Inorg. Chem. 9, 520 (1964).
F. G. Armellini, J. W. Tester, and G. T. Honig, J. Supercritical Fluids 7, 147 (1994).
F. G. Armellini and J. W. Tester, Fluid Phase Equil. 84, 123 (1993).
M. A. Styrikovich, I. Kh. Khibylin, and D. G. Tskhirashvili, Dokl. Akad. Nauk SSSR, 100, 1123 (1955).
M. A. Styrikovich and L.N. Khokhlov, Teploenergetika, 4, 3 (1957).
F. K. Cameron, J. Phys. Chem. 34, 692 (1930).
W. D. Halstead and B. F. Lovey, J. Appl. Chem. Biotechnol. 27, 585 (1977).
E. Posnjak and H. E. Mervin, J. Amer. Chem. Soc. 45, 1965 (1923).
E. A. M. Wetton, Power Ind. Res. 1, 329 (1981).
R. H. Busey, H. F. Holmes, and R. E. Mesmer, J. Chem. Thermodyn. 16, 343 (1984).
J. D. Cline, Limonol. Oceanogr. 14, 454 (1969).
J. Smiltens, J. Chem. Phys. 20, 990 (1952).
J. M. H. Levelt Sengers, in Supercritical Fluid Technology; J. J. Bruno and J. F. Ely, eds; (CRC Press: Boca Raton, FL, 1991), Chap. 1.
J. M. H. Levelt Sengers, J. Supercritical Fluids 4, 215 (1991).
(a) A. H. Harvey, J. Phys. Chem. 94, 932 (1990); (b) A. H. Harvey, J. M. H. Levelt, Sengers, and J. H. Tanger, IV, J. Phys. Chem. 95, 932 (1991).
R. E. Mesmer, D. A. Palmer, and J. M. Simonson, in Activity Coefficients in Electrolyte Solutions, 2nd edn.; K. S. Pitzer, ed.; (CRC Press: Boca Raton, FL, 1991), Chap. 8.
A. H. Harvey, A. P. Peskin, and S. A. Klein, NIST Standard Reference Database 10, Version 2.11 (1997).
J. W. Johnson, E. H. Oelkers, and H. Helgeson, Comp. Geosci. 18, 899 (1992).
W. T. Lindsay, Jr., in The ASME Handbook on Water Technology for Thermal Power Systems, P. Cohen, ed. (Amer. Soc. Mech. Eng., New York, 1989), Chap. 7.
J. L. Oscarson, R. M. Izatt, P. R. Brown, Z. Pawlak, S. E. Gillepsie, and J. J. Christensen, J. Solution Chem. 17, 841 (1988).
G. S. Pokrovski, J Schott, and A. S. Sergeyev, Chem. Geol. 124, 253 (1995).
R. H. Wood, personal communication, March, 2000.
W. L. Marshall and G. M. Begun, J. Chem. Soc. Faraday Trans. II 85, 1963 (1989).
C. Margulis, D. Laria, and R. Fernandez Prini, J. Chem. Soc. Faraday Trans. 92, 2703 (1996).
M. A. Blesa, P. J. Morando, and A. E. Reazzoni, Chemical Dissolution of Metal Oxides, (CRC Press, Boca Raton, Fl, 1992).
D. Postma, Geochim. Cosmochim. Acta 57, 5027 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shvedov, D., Tremaine, P.R. The Solubility of Sodium Sulfate and the Reduction of Aqueous Sulfate by Magnetite under Near-Critical Conditions. Journal of Solution Chemistry 29, 889–904 (2000). https://doi.org/10.1023/A:1005182600421
Issue Date:
DOI: https://doi.org/10.1023/A:1005182600421