Abstract
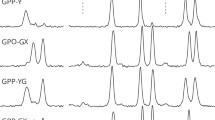
Some details of the backbone dynamics in the collagen-like triple helix is discussed and the role of backbone dynamics in functioning collagen proteins is illustrated. On a series of oligotripeptides synthetic analogs of collagen formation of high-frequency vibrational backbone dynamics and low-frequency nonlinear backbone dynamics upon stepwise elongation of peptide chain have been described using infrared spectroscopy and hydrogen-exchange method. In the fully completed triple helix the level of high-frequency backbone dynamics is regulated firstly by contact interactions of adjacent atoms and chemical bounded groups, while the level of low-frequency large-amplitude backbone dynamics depends mainly on cooperative interactions attributed by conjugation of interpeptide hydrogen bonds. In native collagens the nonlinear large-amplitude dynamics following by non-denaturational micro-unfolding of the triple-helical structure appears to be under the natural selection control delivering an optimal condition for formation, functioning and utilization of collagen fibrils.
Similar content being viewed by others
References
Yakushevich, L.V.: Nonlinear dynamics of biopolymers: theoretical models, experimental data. Quarterly Reviews of Biophysics 26 (1993), 201–223.
Cantor, C.R. and Schimmel P.R: Biophysical chemistry. Part 2. Chapt 7–9. W.H. Freeman and Company. San Francisco. 1979.
Fan, P., Li, M.-H., Brodsky, B. and Baum, J.: Backbone-dynamics of (Pro-Hy-Gly)10 and designed collagen-like triple-helical peptide by 15N NMR relaxation and hydrogen-exchange measurements. Biochemistry 32 (1993), 13299–13309.
Hvidt, A. and Nielsen, S.O.: Hydrogen exchange in proteins. Adv. Prot. Chem. 21 (1966), 287–386.
Lichtenstein, G.I.: Spin-label method in molecular biology. Chapt. 7–8. ‘Nauka’ Publishers. Moscow. 1974.
Kohen, E., Santus, R. and Hirschberg, J.G.: ‘Photobiology’. Chapt.2, 11. Acad. Press. New York, Boston, London. 1994.
Alexandrov, V.Ya.: Cell reactivity and proteins. Chapt. 7–8. ‘Nauka’ Publishers. Leningrad. 1985.
Frazer, R.D.B. and MacRae, T.P.: Conformation in fibrous proteins. Acad. Press. New York. 1973.
Jones, E.Y. and Miller, A.: Analysis of structural design features in collagen. J. Mol. Biol. 218 (1991), 209–219.
Bella, J., Eaton, M., Brodsky, B. and Berman, H.M.: Crystal and molecular structure of collagen-like peptide at 1.9 A resolution. Science 266 (1994), 75–81.
Li, M.-H., Fan, P., Brodsky, B. and Baum, J.: Two-dimensional NMR assignments and conforrnation of (Pro-Hyp-Gly)10 and designed collagen-like triple-helical peptide. Biochemistry 32 (1997), 7377–7387.
Lazarev, Yu.A., Grishkovsky. B.A., Khromova, T.B., Lazareva, A.V. and Grechishko, V.S.: Bound water in collagen-like triple-helical structure. Biopolymers 32 (1992), 189–195.
Lazarev, Yu.A., Lazareva, A.V., Shibnev, V.A. and Esipova N.G.: Infrared spectra and structure of synthetic polytripeptides. Biopolymers 17 (1978), 1197–2214.
Lazarev, Yu.A., Lobachev, V.M., Grishkovski, B.A., Shibnev, V.A., Grechishko, V.S., Figenova, M.P., Esipova, N.G. and Rogulenkova, V.N.: Formation of the collagen-like triple-helical structure in oligopeptides during elongation of the molecular chain. Biopolymers 17 (1978), 1215–1234.
Lazarev, Yu.A., Lazareva, A.V. and Grechishko. V.S.: Influence of molecular chain length on the formation of a collagen-type trihelical complex in solution. The role of water in the formation of the triple helix. Biofizika 42 (1997), 54–67.
Lazarev, Yu.A., Lazareva, A.V., Khromova, T.B. and Grechishko, V.S.: Thermodynamic investigations of the collagen-type trihelical structure in oligotripeptides on successive lengthening of the molecular chain. Biofizika 42 (1997), 326–333.
Lazarev, Yu.A. and Esipova, N.G.: Spectral studies of collagen structure ordering. Dokl. Akad. Nauk. 209 (1973), 478–481.
Privalov, P.L., Tiktopulo, E.I. and Tischenko, V.M.: Stability and mobility of the collagen structure. J. Mol. Biol. 127 (1979), 203–216.
Rigby, B.J.: Thermal transitions in invertebrate collagens and their relation to amino acid content and environmental temperature. In W.G. Grewther (ed.) Symposium on Fibrous Proteins. Butterworths. Sydney. 1968. pp. 217–225.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lazarev, Y., Lazareva, A. & Komarov, V. Backbone Dynamics of Triple-helical Collagen-like Structure. Journal of Biological Physics 24, 217–222 (1999). https://doi.org/10.1023/A:1005180115500
Issue Date:
DOI: https://doi.org/10.1023/A:1005180115500