Abstract
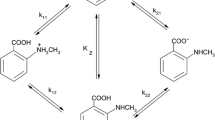
In the framework of our studies on acid=nbase equilibria in systems comprisingsubstituted pyridines and nonaqueous solvents, acid dissociation constants havebeen determined potentiometrically for a variety of cationic acids conjugatedwith pyridine and its derivatives in the polar protophobic aprotic solvent nitromethane. The potentiometric method enabled a check as to whether and to whatextent cationic homoconjugation equilibria of the BH+/B type, as well as cationicheteroconjugation equilibria in BH+/B1 systems without proton transfer, are setup in nitromethane. The equilibrium constants were compared with thosedetermined in water and two other polar protophobic aprotic solvents, propylenecarbonate and acetonitrile. The pK a values of acids conjugate to the N-bases innitromethane fall in the pK a range of 5.84 to 17.67, i.e., 6 to 7 pK a units, onaverage, higher than in water, 1 to 2 units higher than in propylene carbonate,and less than 1 unit lower than in acetonitrile. This means that the basicity ofthe pyridine derivatives increases on going from propylene carbonate throughnitromethane to acetonitrile. Further, it was found that the sequence of the pK achanges of the protonated amines was consistent in all three media, thus providingthe basis for establishing linear correlations among these values. In the majorityof the BH+/B systems in nitromethane, cationic homoconjugation equilibria havebeen established. The cationic homoconjugation constants, log K BHB+, arerelatively low, falling in the range 1.60–2.89. A comparison of the homoconjugationconstants in nitromethane with those in propylene carbonate and acetonitrile showsthat nitromethane is a more favorable solvent for the cationic homoconjugationequilibria than the other two solvents. Moreover, results of the potentiometricmeasurements revealed that cationic heteroconjugation equilibria were not presentin the majority of the BH+/B1 systems in nitromethane. The heteroconjugationconstant could be determined in one system only, with logdiK BHB1 + = 2.56.
Similar content being viewed by others
REFERENCES
J. F. Coetzee, Progr. Phys. Org. Chem. 4, 45 (1967).
N. G. Sellers, P. N. P. Eller, and J. A. Caruso, J. Phys. Chem. 76, 3618 (1972).
B. Brzeziń ski and G. Zundel, J. Chem. Soc. Faraday Trans. II 72, 2127 (1976).
E. Grech, Z. Malarski, and L. Sobczyk, Spectrosc. Lett. 34, 292 (1975).
V. Van Even, and M. C. Haulait-Pirson, J. Solution Chem. 6, 757 (1977).
M. C. Haulait-Pirson and M. De Pauw, J. Phys. Chem. 84, 1381 (1980).
Z. Pawlak, Roczn. Chem. 47, 347 (1973).
J. F. Coetzee and G. R. Padmanabhan, J. Amer. Chem. Soc. 87, 5005 (1965).
Z. Pawlak and R. G. Bates, J. Chem. Thermodyn. 14, 1035 (1982).
J. F. Coetzee, G. R. Padmanabhan, and G. P. Cunningham, Talanta 11, 93 (1964).
I. M. Kolthoff and M. K. Chantooni, Jr., J. Amer. Chem. Soc. 91, 4621 (1969).
Z. Pawlak, S. Kuna, M. Richert, E. Giersz, A. Liwo, and L. Chmurzyń ski, J. Chem. Thermodyn. 26, 483 (1994).
S. Kuna, Z. Pawlak, and M. Tusk, J. Chem. Soc., Faraday Trans I 78 2685 (1982).
A. Wawrzynó w, A. Liwo, E. Kaczmarczyk, and L. Chmurzyń ski, J. Solution Chem. 27 463 (1998).
D. Augustin-Nowacka and L. Chmurzyń ski, Anal. Chim. Acta 381, 215 (1999).
U. Mayer, Coord. Chem. Rev. 21, 159 (1976).
J. Barthel, R. Wachter, and H. H. Gores, Modern Aspects of Electrochemistry, Vol 13, (Plenum, New York, 1979).
J. J. Christensen, L. D. Hansen, and R. M. Izatt, Handbook of Proton Ionization Heats and Related Thermodynamic Quantities (Brigham Young University, Provo, Utah, 1976).
L. Chmurzyń ski, M. Nesterowicz, G. Wawrzyniak, E. Kaczmarczyk, and Z. Warnke, Aust. J. Chem. 49, 931 (1996)
H. Smagowski and G. Wawrzyniak, Pol. J. Chem. 55, 2193 (1981).
L. Chmurzyń ski, A. Wawrzynó w, and Z. Pawlak, Electrochim Acta 35, 665 (1990).
L. Chmurzyń ski, A. Wawrzynó w, and Z. Pawlak, J. Chem. Soc., Faraday Trans. I 85, 4269 (1989).
B. A. Korolev and E. T. Kaszkowskaja, Zh. Obs. Khim. 49, 909 (1979).
J. Kostrowicki and A. Liwo, Comput. Chem. 8, 91,101 (1984)
J. Kostrowicki and A. Liwo, Talanta 37, 645 (1990).
J. Kostrowicki and A. Liwo, Comput. Chem. 11, 195 (1987).
I. M. Kolthoff and M. K. Chantooni, Jr., J. Phys. Chem. 66, 2270 (1968).
A. G. Kozachenko, E. I. Matrosov, and M. I. Kabachnik, Izv. Akad. Nauk SSSR, Ser. Khim., p. 1476 (1976).
L. Chmurzyń ski, Pol. J. Chem. 66, 1165 (1992).
L. Chmurzyń ski, J. Chem. Soc., Faraday Trans. I 87, 1729 (1991).
L. Chmurzyń ski, Anal. Chim. Acta 321, 237 (1996).
H. Smagowski, Acids Strength in Aprotic Non-aqueous Solvents (University of Gdań sk, Gdań sk Poland 1973).
A. J. Parker, Quart. Rev. 16, 163 (1962).
B. A. Korolev and K. A. Osmolovskaa, Zh. Obshch. Khim. 49, 898 (1977).
C. Reichardt, Solvent and Solvent Effects in Organic Chemistry (VCH Publishers, Weinheim, 1988).
L. Chumrzyń ski, Anal Chim. Acta 329, 267 (1996).
L. Chmurzyń ski, A. Liwo, and A. Tempczyk, Z. Naturforsch. 44b, 1263 (1989).
L. Chmurzyń ski, Anal. Chim. Acta 334, 155 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Augustin-Nowacka, D., Chmurzyński, L. Base Equilibria of Substituted Pyridines in Nitromethane. Journal of Solution Chemistry 29, 837–846 (2000). https://doi.org/10.1023/A:1005100331099
Issue Date:
DOI: https://doi.org/10.1023/A:1005100331099