Abstract
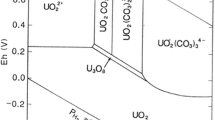
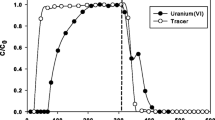
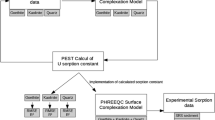
A 300 d solubility study involving two carbonate-rich, uranium-contaminated soils from the Department of Energy's Fernald Environmental Management Project site was conducted to predict the behavior of uranium during on-site remediation of these soils. Geochemical modeling was performed on the aqueous species dissolved from these soils following the solubility measurements to predict the on-site uranium leaching and transport potential. Results showed that the soluble levels of the major components (total uranium, calcium, magnesium, and carbonate) increased continually for the first 4 weeks. After the first 4 weeks, these components either reached a steady-state or continued to increase linearly throughout the study. Soluble uranium levels of both soils and their correlation with alkalinity was strongly mediated by the source term of the contamination. Geochemical modeling predicted and anion exchange experiments confirmed that uranyl-carbonate complexes were the most stable and abundant complexes. Further modeling showed that uranium solubility in these soils and in onsite groundwater wells is controlled by UO2(H2PO4)2, but is also mediated by complexation with carbonate and the oxidation state of the uranium. For assessing the risk related to off-site transport of uranium, it should be recognized that the solubility of uranium-bearing minerals is the critical factor in controlling uranium solubility of these soils rather than sorption/desorption processes as measured by the uranium distribution coefficient (Kd) in these soils.
Similar content being viewed by others
References
Bruno, J. and Sandino, A.: 1989, Mater. Res. Soc. Symp. Proc. 127, 871.
Buck, E. C. Cunnane, J. C. Brown, N. R. and Dietz, N. L.: 1993, Argonne National Laboratory Report No. N94/38, Argonne National Laboratory, Argonne, IL. 52 pp.
Buck, E. C., Brown, N. R. and Dietz, N.L.: 1996, Environ. Sci. & Technol. 30, 81.
Carroll, S. A. and Bruno, J.: 1991, Radiochim. Acta.52-53, 187.
Cunnane, J. C., Gill, V. R., Lee, S. Y., Morris, D. E., Nickelson, M. D. Perry, D. L. and Tidwell, V. C.: 1993, Uranium Soils Integrated Demonstration: Soil Characterization Project Report . FEMP/SUB058, UC702, 72 pp.
Elless, M. P. and Lee, S.Y.: 1994, Oak Ridge National Laboratory Technical Memorandum, ORNL/TM-12848, Oak Ridge National Laboratory, Oak Ridge, TN. 59 pp.
Elless, M. P., Lee. S. Y. and Timpson, M. E.: 1994, Proceedings of Waste Management 94, 3, 2055.
Francis, C. W., Mattus, A. J., Farr, L. L., Elless, M. P., and Lee, S. Y.: 1993, Oak Ridge National Laboratory Technical Memorandum, ORNL/TM12177Oak Ridge National Laboratory, Oak Ridge, TN, 59 pp.
Garrels, R. M. and Christ, C. L.: 1965, Solutions, minerals, and equilibria, Freeman, Cooper, and Company, San Francisco.
Grenthe, 1., Ferri, D., Salvatore, F. and Riccio, G.: 1984, J. Chem Soc. Dalton Trans. 11, 2439.
Ho, C. H. and Miller, N. H.: 1986, J. Colloid Interface Sci. 110, 165.
Hoffman, F. M. and Tripathi, V. S.: 1993, Comput. Geosci. 19, 53.
Langmuir, D.: 1978, Geochim. Cosmochim. Acta, 42, 547.
Lee, S.Y., Elless, M. and Hoffman, F.: 1993, Oak RidgeNational Laboratory Technical Memorandum, ORNL/TM12401, Oak Ridge National Laboratory, Oak Ridge, TN, 33 pp.
Lee, S. Y. and Marsh, Jr, J. D.: 1992, Oak Ridge National Laboratory Technical Memorandum, ORNL/TM11980, Oak Ridge National Laboratory, Oak Ridge, TN, 57 pp.
Milton. G. M. and Brown, R. M.: 1987, Can. J. Earth Sci. 24, 1321.
USDA.: 1979, Soil Survey of Hamilton County, Ohio. Soil Conservation Service, U.S. Department of Agriculture, Washington, D.C.
Westall, J. C., Zachary, J. L., Morel, F. M. M.: 1976, MINEQL.-A computer program for the calculation of chemical equilibrium composition of aqueous systems. Massachusetts Institute of Technology, Cambridge, Mass.
Wilson, J. H., Chernikoff, R., DeMarco, W. D., Francis, C. W., Stebbins, L. L.: 1995, Oak Ridge National Laboratory Technical Memorandum, ORNL/TM12960, Oak Ridge National Laboratory, Oak Ridge, TN, 198 pp.
Author information
Authors and Affiliations
Additional information
now with
Rights and permissions
About this article
Cite this article
Elless, M.P., Lee, S.Y. Uranium Solubility of Carbonate-Rich Uranium-Contaminated Soils. Water, Air, & Soil Pollution 107, 147–162 (1998). https://doi.org/10.1023/A:1004982515941
Issue Date:
DOI: https://doi.org/10.1023/A:1004982515941