Abstract
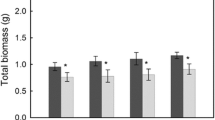
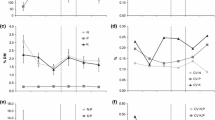
There is much interest in understanding the nature of feedback mechanisms between plants and soil organisms in grazed ecosystems. In this study, we examine the effects of different intensities of defoliation on the growth of three dominant grass species, and observe how these plant responses relate to the biomass and activity of the microbial community in the root zone. Our data show that grassland plants with varying tolerances to grazing have markedly different growth responses to defoliation, and that these responses vary with the intensity of cutting. Defoliation of grasses which are tolerant to grazing, namely Festuca rubra and Cynosurus cristatus, leads to a reduction in root mass and an increase in the allocation of resources to shoots. In contrast, defoliation of a grass with low tolerance to grazing, Anthoxanthum odoratum, had little effect on root mass, but increased the relative allocation of resources below-ground. In all plant species, defoliation led to an increase in soil microbial biomass and C use efficiency in the root zone. This response was greatest in the root zone of A. odoratum and is likely to be related to changes in root exudation pattern following defoliation. The significance of these changes in relation to soil nutrient dynamics and plant nutrient uptake during regrowth require further exploration.
Similar content being viewed by others
References
Anderson J P E and Domsch K H 1985 Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fertil. Soils 1, 81–89.
Bardgett R D, Mawdsley J L, Edwards S, Hobbs P J, Rodwell J S and Davies W J 1999 Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Funct. Ecol. 13, 650–660.
Bardgett R D, Wardle D A and Yeates G W 1998 Linking aboveground and below-ground food webs: how plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 30, 1867–1878.
Chaieb M, Henchi B and Boukhris M 1996 Impact of clipping on root systems of three grasses species in Tunisia. J. Range Manage. 49, 336–339.
Clarholm M 1985 Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol. Biochem. 17, 181–187.
Detling J K, Dyer M I and Winn D T 1979 Net photosynthesis, root respiration and regrowth of Bouteloua gracilis following simulated grazing. Oecologia 41, 127–134.
Donaghy D J and Fulerson W J 1998 Priority allocation of watersoluble carbohydrate reserves during regrowth of Lolium perenne. Grass Forage Sci. 53, 211–218.
Floate M J S 1970a Mineralization of nitrogen and phosphorus from organic materials of plant and animal origin and its significance in the nutrient cycle in grazed upland and hill soils. J. Brit. Grassl. Soc. 25, 295–302.
Floate M J S 1970b Decomposition of organic materials from hill soils and pastures. II. Comparative studies on the mineralization of carbon, nitrogen and phosphorus from plant materials and sheep faeces. Soil Biol. Biochem. 2, 173–185.
Frank D A and Evans RD 1997 Effects of native grazers on grassland N cycling in Yellowstone National Park. Ecology 78, 2238–2248.
Grime J P, Hodgson J G and Hunt R 1988 Comparative Plant Ecology: A Functional Approach to Comkon British Species. Unwin Hyman, London.
Hamilton W E, Giovannini M S, Moses S A, Coleman J S and McNaughton S J 1998 Biomass and mineral element responses of a Serengeti short-grass species to nitrogen supply and defoliation: compensation requires critical [N]. Oecologia 116, 407–418.
Holland J N, Cheng Wand Crossley D A Jr 1996 Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon-14. Oecologia 107, 87–94.
Holland E A and Detling J K 1990 Plant response to herbivory and belowground nitrogen cycling. Ecology 71, 1040–1049.
McNaughton S J 1983 Compensatory plant growth as a response to herbivory. Oikos 40, 329–336.
McNaughton S J, Banyikwa F F and McNaughton M M 1997 Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 278, 1798–1800.
McNaughton S J, Banyikwa F F and McNaughton M M 1998 Root biomass and productivity in a grazing ecosystem: the Serengeti. Ecology 79, 587–592.
Mawdsley J L and Bardgett R D 1997 Continuous defoliation of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) and associated changes in the microbial population of an upland grassland soil. Biol. Fertil. Soils 24, 52–58.
Milchunas D G and Lauenroth W K 1993 Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monog. 63, 327–366.
Oesterheld M and McNaughton S J 1988 Intraspecific variation in the response of Themeda triandra to defoliation: the effect of time of recovery and growth rates on compensatory growth. Oecologia 77, 181–186.
Rodwell J S 1992 Grasslands and Montane Communities. Cambridge University Press.
Ruess R W and McNaughton S J 1987 Grazing and the dynamics and energy regulated microbial processes in the Serengeti grassland. Oikos 49, 101–110.
Sparling G P, West A W, Feltham C W and Reynolds J 1990 Estimation of microbial C by a fumigation extraction method: use on soils of high organic matter content and reassessment of the KEC-factor. Soil Biol. Biochem. 22, 300–309.
Tate K R, Ross D J and Feltham C W 1988 A direct method to estimate soil microbial biomass C: effects of experimental variable and some different calibration procedures. Soil Biol. Biochem. 20, 239–335.
Tracey B F and Frank D A 1998 Herbiore influence on soil microbial biomass and nitrogen mineralization in a northern grassland ecosystem: Yellowstone National Park. Oecologia 114, 556–562.
Vance E D, Brookes P C and Jenkinson D S 1987 An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707.
Wardle D A and Nicholson K S 1996 Synergistic effects of grassland species on soil microbial biomass and activity: implications for ecosystem-level effects of enriched plant diversity. Funct. Ecol. 10, 410–416.
Rights and permissions
About this article
Cite this article
Guitian, R., Bardgett, R.D. Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant and Soil 220, 271–277 (2000). https://doi.org/10.1023/A:1004787710886
Issue Date:
DOI: https://doi.org/10.1023/A:1004787710886