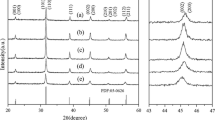
Abstract
Various processes of coprecipitation or crystallization of inorganicsalts of barium and titanium from homogeneous solutions were studiedin this work. In particular, barium hydroxide and barium chloridesalt as well as titanium tetrachloride were used as the startingmaterials for dielectric-tuning homogeneous precipitation in mixedsolvents of isopropanol and water. Hydroxypropylcellulose was used asa steric dispersant. Evaluations of size, shape, and composition ofsynthesized particles were made using scanning electron microscopy,high-temperature X-ray diffractometry, and differential thermalanalysis. Results show that salt concentration, pH, and reaction timeare important in determining the morphology and composition of thefinal powder. The titania particles from dielectric-tuningprecipitation are perfect microspheres with narrow size distribution(near monodispersed), while the particles from barium salts areflake-like, irregular in shape and size. Instead of particlescontaining uniform compositions of barium and titanium compounds,dielectric-tuning coprecipitation yielded powders of two separatedphases, i.e., monodispersed titania microspheres (∼1 μm) coated onbarium chloride salt flakes. Titanium-rich barium titanate wasobtained after calcination of coprecipitated powders. However,preliminary results show that the titania particles obtained bydielectric-tuning precipitation can be hydrothermally converted toBaTiO3 particles that are fully crystallized after calcination above950°C.
Similar content being viewed by others
References
H. Nishizawa and M. Katsube, J. Solid State Chem. 131 (1997) 43.
T. Fukui, C. Sakurai and M. Okuyama, J. Mater. Sci. 32 (1997) 189.
J. C. Niepce and G. Thomas, Solid State Ionics 43 (1990) 69.
P. P. Phule and S. H. Risbud, J. Mater. Sci. 25 (1990) 1169.
J. O. Eckert, Jr., C. C. Hung-houston, B. L. Gersten, M. M. Lencka and R. E. Riman, J. Amer. Ceram. Soc. 79 (1996) 2929.
H. Park, D. Kim and C. Kim, ibid. 80 (1997) 743.
Z. H. Park, H. S. Shin, B. K. Lee and S. H. Cho, ibid. 80 (1997) 1599.
A. V. Ragulya, O. O. Vasylkiv and V. V. Skorokhod, Powder Metallurgy and Metal Ceramics 36 (1997) 170.
S. W. Kim, M. H. Lee, T. Y. Noh and C. Lee, J. Mater. Sci. 31 (1996) 3643.
M. Arima, M. Kakihana, Y. Nakamura, M. Yashima and M. Yoshimura, J. Amer. Ceram. Soc. 79 (1996) 2847.
C. Proust, C. Miot and E. Husson, J. Europ. Ceram. Soc. 15 (1995) 631.
D. Hennings and S. Schreinemacher, ibid. 9 (1992) 41.
D. Hennings, G. Rosenstein and H. Schreinemacher, ibid. 8 (1991) 107.
R. N. Viswanath and S. Ramasamy, NanoStruct. Mater. 8 (1997) 155.
H. Shimooka and M. Kuwabara, J. Amer. Ceram. Soc. 78 (1995) 2849.
S. Takahashi, H. Ohmura, K. Miki, H. Shimooka and M. Kuwabara, J. Ceram. Soc. Jap., Int. Edition 102 (1994) 1182.
K. S. Mazdiyasni, R. T. Dolloff and J. S. Smith, J. Amer. Ceram. Soc. 52 (1969) 523.
M. I. Yanovskaya, N. M. Kotova, I. E. Obvintseva, E. P. Turevskaya, N. YA. Turova, K. A. Vorotilov, L. I. Solov'yova and E. P. Kovsman, Mat. Res. Soc. Symp. Proc. 346 (1994) 15.
P. P. Phule, S. Raghavan and S. H. Risbud, J. Amer. Ceram. Soc. 70 (1987) C108.
K. Kiss, J. Magder, M. S. Vukasovich and R. J. Lockhart, ibid. 49 (1966) 295.
S. S. Flaschen, ibid. 77 (1955) 6194.
M. Ikeda, S.-K. Lee, K. Shinozaki and N. Mizutani, J. Ceram. Soc. Jap., Int. Edition 100 (1992) 674.
A. I. Yanovsky, M. I. Yanovskaya, V. K. Limar, V. G. Kessler, N. YA. Turova and Y. T. Struchkov, J. Chem. Soc., Chem. Commun. (1991) 1605.
P. P. Phule and S. H. Risbud, Mater. Sci. Eng. B3 (1989) 241.
P. Gherardi and E. Matijevič, Colloids Surf. 32 (1988) 257.
E. Shi, C. Xia, W. Zhong, B. Wang and C. Feng, J. Amer. Ceram. Soc. 80 (1997) 1567.
S. Wada, T. Suzuki and T. Noma, J. Ceram. Soc. Jap. 104 (1996) 383.
M. Wu, R. Xu, S. Feng, L. Li, D. Chen and Y. Luo, J. Mater. Sci. 31 (1996) 6201.
C.-T. Xia, E.-W. Shi, W.-Z. Zhong and J.-K. Guo, J. Europ. Ceram. Soc. 15 (1995) 1171.
P. K. Dutta, R. Asiaie, S. A. Akbar and W. Zhu, Chem. Mater. 6 (1994) 1542.
P. K. Dutta and J. R. Gregg, ibid. 4 (1992) 843.
J. A. Kerchner, J. Moon, R. E. Chodelka, A. A. Morrone and J. H. Adair, ACS Symp. Ser. 681 (1998) 106.
F. Dogan, S. O'rourke, M.-X. Qian and M. Sarikaya, Soc. Symp. Proc. 457 (1997) 69.
E. B. Slamovich and I. A. Aksay, J. Amer. Ceram. Soc. 79 (1996) 239.
F. Dogan and M. Sarikaya, Mat. Res. Soc. Proc. 403 (1996) 95.
S. Wada, T. Suzuki and T. Noma, J. Ceram. Soc. Jap., Int. Edition. 103 (1995) 1207.
R. Vivekanandan and T. R. N. Kutty,Powder Technol. 57 (1989) 151.
W. Hertl, J. Amer. Ceram. Soc. 71 (1988) 879.
CH. Beck, W. Hartl and R. Hempelmann, J. Mater. Res. 13 (1998) 3174.
H. Herrig and R. Hempelmann, Mater. Lett. 27 (1996) 287.
D. Sporn, J. Gromann, A. Kaiser, R. Jahn and A. Berger, NanoStruct. Mater. 6 (1995) 329.
L. M. Gan, L. H. Zhang, H. S. O. Chan, C. H. Chew and B. H. Loo, J. Mater. Sci. 31 (1996) 1071.
P. K. Dutta, P. K. Gallagher and J. Twu, Chem. Mater. 4 (1992) 847.
The Aldrich Chemical Company, Inc., “Catalog Handbook of Fine Chemicals,” (1998–1999) pp. 139, 1613.
M. Z.-C. Hu, E. A. Payzant and C. H. Byers, J. Colloid Inter. Sci., 222 (2000) 20.
H. Park, Y. Moon, D. Kim and C. Kim, J. Amer. Ceram. Soc. 79 (1996) 2727.
M. Z.-C. Hu, R. D. Hunt, E. A. Payzant and C. R. Hubbard, ibid. 82 (1999) 2313.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hu, M.ZC., Miller, G.A., Payzant, E.A. et al. Homogeneous (co)precipitation of inorganic salts for synthesis of monodispersed barium titanate particles. Journal of Materials Science 35, 2927–2936 (2000). https://doi.org/10.1023/A:1004718508280
Issue Date:
DOI: https://doi.org/10.1023/A:1004718508280