Abstract
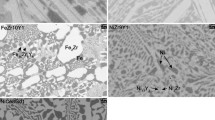
The behavior of liquid tin and its alloys in oxygen at temperature range 600 to 800°C were investigated. Rapid and nearly linear reaction kinetics were observed for pure tin at temperature higher than 700°C. Marker experiments, which determine the mode of mass transport through the scale, and wetting phenomena between the oxide and melts were studied to delineate the reaction mechanism of oxide growth. Moreover, the rates of oxidation of tin were markedly changed by alloying it with small amount of foreign elements. Significantly increased oxidation rates for binary tin alloys containing Mg, Ba, La or Ca were observed. TEM studies indicated that additional growth stresses were introduced into the SnO2 scales by these additions.
Similar content being viewed by others
References
M. S. Newkirk, A. W. Urquhart, H. R. Zwicke and E. Breval, J. Mater. Res. 1(1) (1986) 81–89.
M. S. Newkirk, H. D. Lesher, D. R. White, C. R. Kennedy, A. W. Urquhart and T. D. Claar, Ceram. Eng. Sci. Proc. 8 (1987) 879–885.
M. S. Newkirk and S. F. Dizio, U. S. Patent no. 4,713,360 (1987).
K. Creber, S. D. Poste, M. K. Aghajanian and T. D. Claar, Ceram. Eng. Sci. Proc. 9 (7/8) (1988) 975–982.
D.-W. Yuan, V.-S. Chengn, R.-F. Yan and G. Simkovich, ibid. 15 (4) (1994) 85–94.
D.-W. Yuan, S. G. Song, R.-F. Yan, E. R. Ryba and G. Simkovich, J. Mater. Sci. 34 (6) (1999) 1293–1300.
W. Gruhl and V. Gruhl, Metall. 6 (1952) 177–182.
G. Shimaoka and I. Yamai, J. Chem. Soc. Japan 76 (1955) 965–967.
P. Spinedi, Gazz. Chim. ital. 86 (1956) 579–588.
J. O. Cope, Trans. Faraday Soc. 57 (1961) 493–503.
D. W. Alymore, S. J. Gregg and W. B. Jepson, J. Electrochem. Soc. 106 (1959) 1010–1013.
J. C. Nover and F. D. Richardson, Trans. Inst. Min. Metall. 81 (1972) c63–c68.
C. Wagner, “Atom Movements” (ASM, Cleveland, OH, 1951).
T. Shuey, “Semiconducting Ore Minerals” (Elsevier, New York, 1975).
W. E. Boggs, R. H. Kochik and G. E. Pellissier, J. Electrochem. Soc. 110 (1963) 4–11.
K. Hauffe and C. Gensch, Z. Physik. Chem. 195 (1950) 116–128.
O. Kubaschewski, E. Evans and C. B. Alcock, “Metallurgical Thermochemistry” (Pergamon Press, New York, 1967) pp. 304–363.
V. R. Howes and C. N. Richardson, Corr. Sci. 9 (1969) 385–394.
J. Stringer, ibid. 10 (1970) 513-543.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yuan, DW., Yan, RF. & Simkovich, G. Rapid oxidation of liquid tin and its alloys at 600 to 800°C. Journal of Materials Science 34, 2911–2920 (1999). https://doi.org/10.1023/A:1004699705451
Issue Date:
DOI: https://doi.org/10.1023/A:1004699705451