Abstract
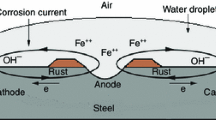
Polyphenylenesulphide (PPS) polymer was applied as a sealing material to flame-sprayed nickel–aluminium (Ni–Al) coatings to protect the interior surfaces at the ends where the mild carbon steel (MCS) heat-exchanger tubes are jointed to the tubesheet. The aim of applying PPS is to prevent their corrosion, oxidation and abrasive wear, in a low pH, hypersaline brine geothermal environment at 200°C under a hydrothermal pressure of 1.6 MPa. Although the Ni–Al coatings had an excellent thermal conductivity and a good wear-resistance, the inherent open structure of these coatings allowed the hot brine to permeate them easily under such pressure, causing the development of corrosion-induced stress cracks in the MCS. Furthermore, under these conditions, the coatings underwent oxidation with the formulation of Al2O3 as the major scale compound and NiO as the minor one. PPS sealant was used to solve these problems. However, one major drawback of PPS was its susceptibility to oxidation reaction with hot brine. This reaction not only incorporated more oxygen into the PPS, generating a sulphide → sulphone transformation within PPS, but also it caused the decomposition of PPS, yielding polychloroaryl compound, and sodium sulphate, and also evolving SO2 gases. The SO2 gases had a chemical affinity for oxide scales in Ni–Al, forming water-soluble Al2(SO4)3 and NiSO4 salt reaction products at the PPS/Ni–Al interfaces. Despite the occurrence of such oxidation damage in PPS, an exposure for 14 days showed that there was no development of corrosion-caused cracks at the interfaces between the underlying steel and Ni–Al, nor was a striking oxidation of the sealed coating panels.
Similar content being viewed by others
References
J. J. Fontana, W. Ream and H. C. Cheng, in “5th International Congress on Polymer in Concrete”, edited by B. W. Staynes (Brighton Polytechnic, Brighton, 1987) p. 399.
V. Hassani and E. Hoo,“Results of Field Testing on Heat Exchangers with Polymer Concrete lining”, National Renewable Energy Laboratory (NREL) USA (1995).
M. Kindschi, personal communications, Hughes-Anderson Heat Exchangers, Inc. July (1997).
S. Sampath, G. A. Banke, H. Herman and S. Rangaswamy, Surf. Eng. 5 (1989) 293.
D. N. Ronald, R. B. Randy and M. V. Nathal,“Physical and Mechanical Metallurgy of NiAl”, National Aeronautics and Space Administration (NASA) Technical Paper 3398, April (1994).
M. S. Reisch, Chem. Eng. News 71 (1993) 24.
M. P. Seah and W. A. Dench, Surf. Interface Anal. 1 (1979) 2.
D. E. Passoja, H. F. Hillery, T. G. Kinisky, H. A. Six, W. T. Jansen and J. A. Taylor, J. Vac. Sci. Technol. 21 (1982) 933.
K. S. Kim and R. E. Davis, J. Electron Spectrosc. 1 (1973) 251.
E. W. A. Yound, J. C. Riviere and L. S. Welch, Appl. Surface Sci. 28 (1987) 71.
N. S. Mcintyre and D. G. Zetaruk, Anal. Chem. 49 (1977) 1521.
J. Riga, J. P. Boutique, J. J. Verbist, in “Physicochemical Aspects of Polymer Surfaces”, edited by K. L. Mittal (Plenum Press, New York, 1983) p. 45.
B. J. Lindberg, K. Hamrin, G. Johnasson, U. Gelius, A. Fahlman, C. Nordling and K. Sieg-Bahn, Phys. Scripta 1 (1970) 286.
C. E. Mixan and J. B. Lawbert, J. Org. Chem. 38 (1975) 1350.
N. H. Turner, J. S. Murday and D. E. Ramaker, Anal. Chem. 52 (1980) 84.
R. W. Lenz and W. K. Carrington, J. Polym. Sci. 41 (1959) 333.
Idem, ibid. 43 (1960) 167.
D. T. Clark, D. Kilcast, W. K. R. Musgrave, J. Chem. Soc. Chem. Commun. 4 (1971) 517.
T. Sugama and N. R. Carciello, J. Adhes. Adhesive 11 (1997) 97.
R. B. Shalvoy and P. J. Reucroft, J. Vac. Sci. Technol. 16 (1979) 567.
F. Mansfeld, M. N. Kendig and S. Tsai, Corrosion 38 (1982) 570.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sugama, T. Polyphenylenesulphide-sealed Ni–Al coatings for protecting steel from corrosion and oxidation in geothermal environments. Journal of Materials Science 33, 3791–3803 (1998). https://doi.org/10.1023/A:1004678831064
Issue Date:
DOI: https://doi.org/10.1023/A:1004678831064