Abstract
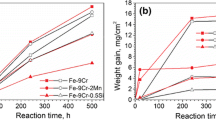
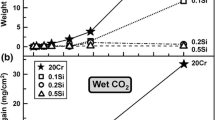
The corrosion behavior of Fe–22Mo–10Al (a/o, atom %),Fe–20.5Mo–15.7Al, and Fe–10Mo–19Al was examined inflowing H2/H2S gases of 4 Pa sulfur partial pressureat 900°C. Al2O3 was stable on all the alloys inthe atmospheres investigated. Fe–22Mo–10Al andFe–20.5Mo–15.7Al reacted slowly, following the parabolic ratelaw. Multilayered reaction products were formed on these alloys and it isuncertain which layer(s) provided the protection. Fe–10Mo–19Alreacted even more slowly, exhibiting two-stage parabolic kinetics. Duringthe early stage of this alloy's reaction, a preferential reaction zone,consisting of an oxide mixture, possibly Al2O3+FeAl2O4,and nonreacting Fe3Mo2, provided the protection. Duringthe later reaction stage, the formation of a continuous, externalAl2O3 layer further decreased the alloy reaction rate.
Similar content being viewed by others
REFERENCES
K. Natesan, J. Met. 43, 61 (1991).
F. Gesmundo, Proc. Conf. High Temp. Mater. Power Eng. I, p. 67 (1990).
K. Natesan, Corrosion-NACE 41, 646 (1985).
W. J. Lochmann, Metallur. Trans. A 9A, 175 (1978).
K. N. Strafford and P. K. Datta, Mater. Sci. Technol. 5, 765 (1989).
H. S. Hsu, Oxid. Met. 18, 213 (1987).
M. F. Rothman, Met. Mater. 8, 93 (1992).
C. Y. Antony, G. Y. Lai, and S. K. Srivastava, Waste Management 11, 71 (1991).
S. Mrowec and K. Przybylski, High Temp. Mater. Process. 6, 1 (1984).
D. J. Young, Rev. High-Temp. Mater. 6, 299 (1980).
H. Habazaki, J. Dabek, K. Hashimoto, S. Mrowec, and M. Danielewski, Corros. Sci. 34, 183 (1993).
K. Przybylski, T. Narita, and W. W. Smeltzer, Oxid. Met. 38, 1 (1992).
G. Southwell and D. J. Young, Oxid. Met. 36, 409 (1991).
D. J. Young and J. P. Orchard, Can. Metallur. Quart. 30, 227 (1991).
W. H. Cheung and D. J. Young, Oxid. Met. 36, 15 (1991).
H. L. Du, P. K. Datta, J. S. Gray, and K. N. Strafford, Oxid. Met. 39, 107 (1993).
Yisheng Chen, Ph.D. Dissertation, University of New South Wales, Australia, 1993.
Ge Wang, D. L. Douglass, and F. Gesmundo. Oxid. Met. 35, 349 (1991).
Yisheng Chen, D. J. Young, and S. Blairs, Oxid. Met. 40, 433 (1993).
E. M. Fryt, V. S. Bhide, W. W. Smeltzer, and J. S. Kirkaldy, J. Electrochem. Soc. 126, 683 (1979).
Yisheng Chen, D. J. Young, and S. Blairs, Oxid. Met. 40, 245 (1993).
Yisheng Chen, D. J. Young, and S. Blairs, Corros. Sci. 36, 401 (1994).
Yisheng Chen, D. J. Young, and S. Blairs, Oxid. Met. 42, 485 (1994).
O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry (Pergamon Press, Oxford, 1979).
M. J. Ferrante, J. M. Stuve, H. C. Ho, and R. R. Brown, High Temp. Sci. 14, 91 (1981).
N. Birks, Proc. Symp. Properties High Temp. Alloys, Z. A. Foroulis and F. S. Pettit, eds. (Electrochemical Society, Inc., Pennington, NJ, 1976), p. 215.
T. C. Tiearney, Jr. and K. Natesan, Oxid. Met. 17, 1 (1982).
H. Habazaki, J. Dabek, K. Hashimoto, S. Mrowec, and M. Danielewski, Corros. Sci. 34, 183 (1993).
P. C. Patnaik and W. W. Smeltzer, J. Electrochem. Soc. 131, 2688 (1984).
O. Kubaschewski and R. Schmid-Fetzer, in Ternary Alloys, Vol. 5, G. Petzow and G. Effenberg, eds. (VCH Verlagshesellschaft, Weinheim, Germany, 1992), p. 325.
A. C. Turnock and H. P. Eugster, J. Petrol. 3, 533 (1962).
F. A. Elrefaie and W. W. Smeltzer, Metallur. Trans. B 14B, 85 (1983).
P. J. Smith and W. W. Smeltzer, Oxid. Met. 28, 291 (1987).
F. H. Stott, F. M. F. Chong, and C. A. Stirling, Proc. Intern. Congr. Met. Corros. 2, 1 (1984).
K. Ohla, S. W. Kim, H. Fischmeister, and E. Fromm, Oxid. Met. 36, 379 (1991).
S. W. Kim, K. Ohla, H. Fischmeister, and E. Fromm, Oxid. Met. 36, 395 (1991).
Z. E. Majid and M. Lambertin, Oxid. Met. 27, 333 (1987).
S. Sheybany and D. L. Douglass, Oxid. Met. 29, 307 (1988).
J. K. R. Weber and M. G. Hocking, Oxid. Met. 32, 1 (1989).
S. Taniguchi and T. Shibata, Oxid. Met. 32, 391 (1989).
S. Taniguchi, T. Shibata, and T. Niida, Oxid. Met. 34, 277 (1990).
S. Sheybany and D. L. Douglass, Oxid. Met. 30, 433 (1988).
P. Singh and N. Birk, Oxid. Met. 19, 37 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, R.Y., Young, D.J. & Blairs, S. The Corrosion Behavior of Sulfidation-Resistant Fe–Mo–Al Alloys in H2/H2S Atmospheres at 900°C. Oxidation of Metals 54, 103–120 (2000). https://doi.org/10.1023/A:1004654713700
Issue Date:
DOI: https://doi.org/10.1023/A:1004654713700