Abstract
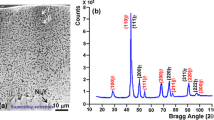
The structure and chemistry of the thermally grown oxide scale in a thermalbarrier coating having an electron beam-physical vapor depositedyttria-partially stabilized zirconia (YPSZ) topcoat and aplatinum–nickel–aluminide (Pt–Ni–Al) bondcoat werestudied using transmission electron microscopy. The scale consisted ofhexagonal aluminum oxide (α-Al2O3) in both as-coated and thermallycycled specimens; no metastable Al2O3 polymorphs were observed. In as-coatedspecimens, the scale's grains had a fine, columnarmorphology. ZrO2-rich dispersoids were observed both intra- andintergranularly throughout the scale. Thermally cycled specimens had aduplex scale structure: one band of grains adjacent to the YPSZ had anequiaxed morphology and contained ZrO2-rich dispersoids; a second band ofdispersoid-free grains adjacent to the bondcoat had a coarse, columnarmorphology. Porosity and cracks were associated with the interface betweenthe bands. The formation of the banded morphology and the cracking areproposed to be associated with the presence of the ZrO2-rich dispersoids.
Similar content being viewed by others
REFERENCES
A. S. Brown, Aerospace Amer. 34, 22 (1996).
A. Maricocci, A. Bartz, and D. Wortman, J. Thermal Spray Technol. 6, 193 (1997).
E. C. Duderstadt and B. A. Nagaraj, U.S. Patent No. 5,238,752, 24 August 1993.
J. Cheng, E. H. Jordan, B. Barber, and M. Gell, Acta Mater. 46, 5839 (1998).
S. M. Meier, D. M. Nissley, and K. D. Sheffler, NASA CR 189111 (1991).
E. J. Felton and F. S. Pettit, Oxid. Met. 10, 189 (1976).
J. Schaeffer, G. M. Kim, G. H. Meier, and F. S. Pettit, in The Role of Active Elements in the Oxidation Behavior of Higher Temperature Metals and Alloys, E. Lang, ed. (Elsevier Applied Science, New York, 1989), pp. 231-267.
M. Gell, K. Vaidyanathan, B. Barber, J. Cheng, and E. Jordan, Metall. Trans. 30A, 427 (1999).
I. Levin and D. Brandon, J. Amer. Ceram. Soc. 81, 1995 (1998).
J. Doychak, J. L. Smialek, and T. E. Mitchell, Metall. Trans. 20A, 499 (1989).
F. H. Stott, Mater. Sci. Forum 254, 19 (1997).
J. Peters and H. Grabke, Werkst. Korros. 35, 385 (1984).
B. A. Pint, J. R. Martin, and L. W. Hobbs, Oxid. Met. 39, 167 (1993).
F. A. Golightly, F. H. Stott, and G. C. Wood, Oxid. Met. 10, 163 (1976).
Y. H. Sohn, R. R. Biederman, and R. D. Sisson, Jr., J. Mater. Eng. Perform. 3, 55 (1994).
P. K. Wright, Mater. Sci. Eng. A245, 191 (1998).
B. A. Pint, I. G. Wright, W. Y. Lee, Y. Zhang, K. Prüssner, and K. B. Alexander, Mater. Sci. Eng. A245, 201 (1998).
O. Unal, T. E. Mitchell, and A. H. Heuer, J. Amer. Ceram. Soc. 77, 984 (1994).
K. Fritscher, M. Schmücker, C. Leyens, and U. Schulz, Mater. Sci. Forum 254, 965 (1997).
J. A. Conner, D. A. Moore, and R. D. Wustman, ASME 91-GT-379, 1991.
M. R. Brickey and J. L. Lee, Microsc. Microanalysis 6, 231 (2000).
N. J. Zaluzec, Electron Microsc. Soc. Amer. Bull. 14, 67 (1984).
N. J. Zaluzec, Electron Microsc. Soc. Amer. Bull. 14, 61 (1984).
N. J. Zaluzec, Electron Microsc. Soc. Amer. Bull. 15, 67 (1985).
N. Birks, G. H. Meier, and F. S. Pettit, J. Mater. 46, 42 (1994).
J. Smialek, and R. Gibala, in High Temperature Corrosion, R. A. Rapp, ed. (National Association of Corrosion Engineers, Houston, TX, 1993), pp. 274-283.
J. L. Smialek, Metall. Trans. 9A, 309 (1978).
F. N. Rhines and J. S. Wolf, Metall. Trans. 1, 1701 (1970).
B. A. Pint, A. J. Garratt-Reed, and L. W. Hobbs, in Microscopy of Oxidation 2, S. B. Newcomb and M. J. Bennett, eds. (Institute of Metals, London, U.K., 1993), pp. 463-475.
A. M. Alper, R. N. McNally, and R. C. Doman, Amer. Ceram. Soc. Bull. 43, 643 (1964).
T. Kosmac, S. Kolar, and M. Trontelj, in Science and Technology of Zirconia II, N. Claussen, M. Rühle, and A. H. Heuer, eds. (The American Ceramic Society, Columbus, OH, 1983), pp. 546-552.
H. G. Scott, J. Mater. Sci. 10, 1527 (1975).
C. Wagner, Z. Phys. Chem. 21B, 25 (1933).
D. A. Jones, Principles and Prevention of Corrosion, 2nd edn. (Prentice Hall, Upper Saddle River, NJ, 1996), p. 429.
J. Doychak and M. Rühle, Oxid. Met. 31, 431 (1989).
I. M. Lifshitz and V. V. Slyozov, J. Phys. Chem. Solids 19, 35 (1961).
R. A. Miller, J. Amer. Ceram. Soc. 67, 517 (1984).
A. M. Freborg, B. L. Ferguson, W. J. Brindley, and G. J. Petrus, Mater. Sci. Eng. A245, 182 (1998).
L. Lelait, S. Alpérine, C. Diot, and M. Mévrel, Mater. Sci. Eng. A121, 475 (1989).
S. Alpérine and L. Lelait, ASME 92-GT-317, 1992.
S. Stecura, NASA TM-78976, 1978.
M. Ohring, The Materials Science of Thin Films (Academic Press, New York, 1992), p. 552.
J. Doychak, J. L. Smialek, and C. A. Barrett, in Oxidation of High-Temperature Intermetallics, T. Grobstein and J. Doychak, eds. (The Minerals, Metals & Materials Society, Warrendale, PA, 1988), pp. 41-55.
A. van Hook, Crystallization: Theory and Practice (Reinhold, New York, 1961), pp. 1-44.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brickey, M.R., Lee, J.L. Structural and Chemical Analyses of a Thermally Grown Oxide Scale in Thermal Barrier Coatings Containing a Platinum–Nickel–Aluminide Bondcoat. Oxidation of Metals 54, 237–254 (2000). https://doi.org/10.1023/A:1004646227870
Issue Date:
DOI: https://doi.org/10.1023/A:1004646227870