Abstract
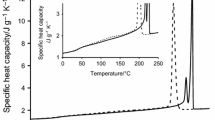
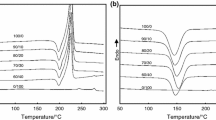
Differential scanning calorimetry (DSC) studies demonstrate that the multiple melting behavior observed in both R and S enantiomorphs of poly(epichlorohydrin), their equimolar blend and the stereoblock copolymer form follows two distinctly different patterns, depending on the crystallization conditions. Isothermal crystallization at large undercoolings results in primary crystallites which during the heating scan undergo a process of melting/recrystallization and final melting, evidenced by a triple melting peak endotherm with the shape and position of the highest melting peak strongly dependent on scanning rate. By comparison, isothermal crystallization at small undercoolings yields primary and secondary crystallized material which melts with a double peak endotherm, the shape of which depends strongly on the crystallization time, with no indication of reorganization during the scan. These melting behavior observations support previous suggestions about the role of enantiomorphism in the crystallization of the stereoblock copolymer. Characteristic slow overall rates of crystallization of poly(epichlorohydrin) make this polymer an ideal subject for the study and refinement of multiple melting in polymers.
Similar content being viewed by others
References
H. E. Bair, R. Salovey, T. W. Huseby, Polymer 8 (1967) 9.
R. C. Roberts, J. Polym. Sci., Polym. Lett. 8 (1970), 381.
P. J. Lemstra, T. Kooistra and G. Challa, J. Polym. Sci., Polym. Phys. Ed. 10 (1972) 823.
P. J. Lemstra, A. J. Schouten and G. Challa, ibid. 12 (1974) 1565.
Z. Pelzbauer and R. J. St. John Manley, J. Polym.Sci., Part A-2 8 (1970) 649.
J. T. Yeh and J. Runt, J. Polym. Sci., Polym. Phys. Ed. 27 (1989) 1543.
J. Rodriguez-Arnold, A. Zhang, S. Z. D. Cheng, A. Lovinger, E. T. Hsieh, P. Chu, T. W. Johnson, K. G. Honnell, R. G. Geerts, S. J. Palacki, G. R. Hawley and M. B. Welch, Polymer 35 (1994) 1884.
V. Caldas, G. R. Brown, R. S. Nohr and J. G. Macdonald, J. Polym. Sci, Polym. Phys. Ed. 34 (1996) 2085.
E. G. Lovering and D. C. Wooden, J. Polym. Sci., Polym. Phys. Ed. 7 (1969) 1639.
W. M. Prest, Jr., and D. J. Luca, J. Appl. Phys. 46 (1975) 4136.
J. S. Chung and P. Cebe, Polymer 33 (1992) 2312.
Idem., ibid. 33 (1992) 2325.
T. P. Russell and J. T. Koberstein, J. Polym. Sci., Polym. Phys. Ed. 23 (1985) 1109.
D. J. Blundell, Polymer 28 (1987) 2248.
M. P. Divito, R. B. Cassel, M. Margulies and S. Goodkowsky, Amer. Lab. August (1995) 28.
K. L. Singfield and G. R. Brown, Macromolecules 28 (1995) 1290.
K. L. Singfield, J. M. Klass and G. R. Brown, ibid. 28 (1995) 8006.
P. J. Holdsworth and A. Turner-Jones, Polymer 12 (1971) 195.
R. C. Roberts, Polymer 10 (1969) 117.
G. E. Sweet and J. P. Bell, J. Polym. Sci., Part A-2 10 (1972) 1273.
P. B. Rim and J. P. Runt, Macromolecules 16 (1983) 762.
Y. Lee and R. S. Porter, ibid. 20 (1987) 1336.
H. Janeczek, B. Trzebicka and E. Turska, Polym. Commun. 28 (1987) 123.
D. C. Bassett, R. H. Olley and I. A. M. Al-Raheil, Polymer 29 (1988) 1745.
R. Verma, H. Marand and B. Hsiao, Macromolecules 29 (1996) 7767.
S. Z. D. Cheng, M. Y. Cao and B. Wunderlich, ibid. 19 (1986) 1868.
I. A. Al-Raheil and A. M. Qudah, A Polym. Int. 37 (1995) 249.
F. J. Medellin-Rogriguez and P. J. Phillips, Macromolecules 29 (1996) 7491.
Rights and permissions
About this article
Cite this article
Singfield, K.L., Brown, G.R. Multiple melting behavior of poly(epichlorohydrin) enantiomorphs. Journal of Materials Science 34, 1323–1331 (1999). https://doi.org/10.1023/A:1004502232614
Issue Date:
DOI: https://doi.org/10.1023/A:1004502232614