Abstract
Here we describe a simple method for pulse-labeling tree seedlings with 13CO2(gas), and then apply the method in two related experiments: t (i) comparison of carbon allocation patterns between t Betula papyrifera Marsh. and t Pseudotsuga menziesii (Mirb.) Franco, and t (ii) measurement of one-way belowground carbon transfer from t B. papyrifera to t P. menziesii. Intraspecific carbon allocation patterns and interspecific carbon transfer both influence resource allocation, and consequently development, in mixed communities of t B. papyrifera and t P. menziesii.
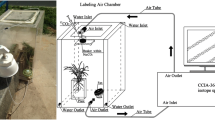
In preparation for the two experiments, we first identified the appropriate 13CO2(gas) pulse-chase regime for labeling seedlings: a range of pulse (100-mL and 200-mL 99 atom%13 CO2(gas)) and chase (0, 3 and 6 d) treatments were applied to one year-old t B. papyrifera and t P. menziesii seedlings. The amount of 13CO2 fixed immediately after 1.5 h exposure was greatest for both t B. papyrifera (40.8 mg excess 13C) and t P. menziesii (22.9 mg excess 13C) with the 200-mL pulse, but higher 13C loss and high sample variability resulted in little difference in excess13 C content between pulse treatments after 3 d for either species. The average excess 13C root/shoot ratio of t B. papyrifera and t P. menziesii changed from 0.00 immediately following the pulse to 0.61 and 0.87 three and six days later, which reflected translocation of 75% of fixed isotope out of foliage within 3 d following the pulse and continued enrichment in fine roots over 6 d. Based on these results, the 100-mL CO2(gas) and 6-d chase were considered appropriate for the carbon allocation and belowground transfer experiments.
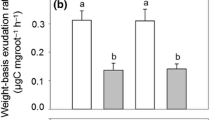
In the carbon allocation experiment, we found after 6 d that t B. papyrifera allocated 49% (average 9.5 mg) and t P. menziesii 41% (average 5.8 mg) of fixed isotope to roots, of which over 55% occurred in fine roots in both species. Species differences in isotope allocation patterns paralleled differences in tissue biomass distribution. The greater pulse labeling efficiency of t B. papyrifera compared to t P. menziesii was associated with its two-fold and 13- fold greater leaf and whole seedling net photosynthetic rates, respectively, 53% greater biomass, and 35% greater root/shoot ratio.
For the carbon transfer experiment, t B. papyrifera and t P. menziesii were grown together in laboratory rootboxes, with their roots intimately mingled. A pulse of 100 mL13 CO2(gas) was applied to paper birch and one-way transfer to neighboring t P. menziesii was measured after 6 d. Of the excess 13C fixed by t B. papyrifera, 4.7% was transferred to neighboring t P. menziesii, which distributed the isotope evenly between roots and shoots. Of the isotope received by t P. menziesii, we estimated that 93% was taken up through belowground pathways, and the remaining 7% taken up by foliage as13 CO2(gas) respired by t B. papyrifera shoots. These two experiments indicate that t B. papyrifera fixes more total carbon and allocates a greater proportion to its root system than does t P. menziesii, giving it a competitive edge in resource gathering; however, below-ground carbon sharing is of sufficient magnitude that it may help ensure co-existence of the two species in mixed communities.
Similar content being viewed by others
References
Alpert P, Warembourg F R and Roy J 1991 Transport of carbon among connected ramets of Eichhornia crassipes(Pontederiaceae) at normal and high levels of CO2. Am. J. Bot. 78, 1459- 1466.
Berg J D, Hendrix P F, Cheng WX and Dillard A L 1991 A labeling chamber for 13C enrichment of plant tissue for decomposition studies. Agric. Ecosys. Environ. 34, 421-425.
Boutton TW 1991 Stable carbon isotope ratios of natural materials. I. sample preparation and mass spectrometric analysis. InCarbon Isotope Techniques. Eds. D C Coleman and B Fry. pp 155-172 Academic Press Inc., Harcourt Brace Jovanovich Publishers, San Diego, CA.
Buchanan DL, Nakao A and Edwards G 1953 Carbon isotope effects in biological systems. Science. 117, 541-545.
Craig H 1954 Carbon 13 in plants and the relationships between carbon 13 and carbon 14 variations in nature. J. Geol. 62, 115- 149.
Dosskey M G, Linderman R G and Boersma L 1990 Carbon-sink stimulation of photosynthesis in Douglas-fir seedlings by some ectomycorrhizas. New Phytol. 115, 269-274.
Ehleringer J R 1991 13C/12C fractionation and its utility in terrestrial plant studies. InCarbon Isotope Techniques. Eds. D C Coleman and B Fry. pp 187-200. Academic Press Inc., Harcourt Brace Jovanovich Publishers, San Diego.
Finlay R D and Read D J 1986 The structure and function of the vegetative mycelium of ectomycorrhizal plants. I. Translocation of 14C-labeled carbon between plants interconnected by a common mycelium. New Phytol. 1986, 143-156.
Finlay R and Söderström B 1992 Mycorrhiza and carbon flow to the soil. InMycorrhizal Functioning: an Integrative Plant-Fungal Process. Ed. M F Allen. pp 134-162. Routledge, Chapman and Hall, Inc., New York.
Francis R and Read D J 1984 Direct transfer of carbon between plants connected by vesicular- arbuscularmycorrhizalmycelium. Nature 307, 53-56.
Geiger D R 1986 Processes affecting carbon allocation and partitioning among sinks. InPlant Biology: 1, Phloem Transport. Eds. J Cronshaw, WL Lucas and R T Giaquinta. pp 375-388. Alan R. Liss, New York.
Gordon A J, Ryle g J A and Powell C E 1977 The strategy of carbon utilization in uniculm barley. I. The chemical fate of photosynthetically assimilated 14C. J. Exp. Bot. 107, 1258-1269.
Gunderson C A and Wullschleger S D 1994 Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photo. Res. 39, 369-388.
Herold A 1980 Regulation of photosynthesis by sink activity-the missing link. New Phytol. 86, 131-144.
Hollinger DY 1987 Gas exchange and dry matter allocation responses to elevation of atmospheric CO2 concentration in seedlings of three tree species. Tree Physiol. 3, 193-202.
Hutchings M J and Bradbury I K 1986 Ecological perspectives on clonal perennial herbs. BioScience 36, 178-182.
Jonsdottir I S and Callaghan T V 1989 Intraclonal translocation of ammonium and nitrate nitrogen in Carex bigelowiiTorr. ex Schwein. using 15N and nitrate reductase assays. New Phytol. 114, 419-428.
Kim HY and Suzuki Y 1989 Changes in assimilated 13C distribution and soluble acid invertase activity of Zinnea elegansinduced by uniconazol, an inhibitor of gibberellin biosynthesis. Plant Physiol. 90, 316-321.
Kinerson R S, Ralston C W and Wells C G 1977 Carbon cycling in a loblolly pine plantation. Oecologia 29, 1-10.
Kouchi H and Yoneyama T 1984 Dynamics of carbon photosynthetically assimilated in nodulated soya bean plants under steady state conditions. I. Development and application of 13CO2 assimilation system at a constant 13C abundance. Ann. Bot. 53, 875-882.
Larcher W 1983 Physiological Plant Ecology, second edition. Springer-Verlag, New York. 303 p.
Lloyd D A, Angove K, Hope G and Thompson C 1990 A guide to site identification and interpretation for the Kamloops Forest Region. Land Management Handbook. ISSN 0229-1622; no. 23. B.C. Ministry of Forests, Victoria.
Miller R F and Rose J A 1992 Growth and carbon allocation of Agropyron desertorumfollowing autumn defoliation. Oecologia 89, 482-486.
Miller S L, Durall DM and Rygiewicz P T 1989 Temporal allocation of 14C to extramatrical hyphae of ectomycorrhizal ponderosa pine seedlings. Tree Physiol. 5, 239-249.
Morrison D J, Wallis G W and Weir L C 1988 Control of Armillariaand Phellinusroot diseases: 20-year results from the Skimikin stump removal experiment. Information Report BC-X- 302. Canadian Forest Service, Pacific Forestry Centre, Victoria.
NaDelhoffer K J and Fry B 1988 Controls on natural nitrogen-15 and carbon-13 abundances in forest soil organic matter. Soil Sci. Soc. Am. J. 52, 1633-1640.
Neilson R E 1977 A technique for measuring photosynthesis in conifers by 14CO2 uptake. Photosynthetica 11, 241-250.
Newman E 1988 Mycorrhizal links between plants: their functioning and ecological significance. Adv. Ecol. Res. 18, 243-270.
O'Leary MH, Madhavan S and Paneth P 1992 Physical and chemical basis of carbon isotope fractionation in plants. Plant Cell Environ. 15, 1099-1104.
Paul E A and Kucey R M N 1981 Carbon flow in plant microbial associations. Science 213, 473-474.
Pearcy RW, Bjorkman O, Caldwell M M, Keeley J E, Monson R K and Strain B R 1987 Carbon gain by plants in natural environments. BioScience 37, 21-28.
Perry D A 1994 Forest Ecosystems. Johns Hopkins University Press, Baltimore. 649 p.
Perry D A, Bell T and Amaranthus M P 1992 Mycorrhizal fungi in mixed-species forests and other tales of positive feedback, redundancy and stability. InThe Ecology of Mixed-Species Stands of Trees. Eds. M G R Cannell, D C Malcom and P A Robertson. pp 151-179. British Ecological Society Special Publication No. 11. University Press, Cambridge.
Pettersson R and McDonald A J S 1992 Effects of elevated carbon dioxide concentration on photosynthesis and growth of small birch plants (Betula pendulaRoth.) at optimal nutrition. Plant Cell Environ. 15, 911-919.
Ranney T G, Bir R E and Skroch W A 1991 Comparative drought resistance among six species of birch (Betula): influence of mild water stress on water relations and leaf gas exchange. Tree Physiol. 8, 351-360.
Read D J, Francis R and Finlay R D 1985 Mycorrhizal mycelia and nutrient cycling in plant communities. InEcological Interactions in Soil: Plants, Microbes and Animals. Eds. A H Fitter, D Atkinson, D J Read and M B Usher. pp 193-217. British Ecological Society Special Publication No.4. Blackwell Scientific Publications, Oxford.
Rygiewicz P T, Miller S L and Durall D M 1988 A root-mycocosm for growing ectomycorrhizal hyphae from host roots while maintaining symbiotic integrity. Plant Soil 109, 281-284.
Sachs D L 1995 Simulation of the growth of mixed stands of Douglas-fir and paper birch using the FORECAST model. InSilviculture of Temperate and Boreal Broadleaved-Conifer Species: Proceedings of a Workshop held February 28 - March 1, 1995 in Richmond, B.C. Eds. P G Comeau and K D Thomas. pp 152- 158. Land Management Handbook 36. B.C. Ministry of Forests, Victoria.
Simard S W 1990 A retrospective study of competition between paper birch and planted Douglas-fir. FRDA Report. ISSN 0835- 0752; 147. Forestry Canada and B.C. Ministry of Forests, Victoria. 18 p.
Simard SW 1995 Interspecific carbon transfer in ectomycorrhizal tree species mixtures. Ph.D. Dissertation. Oregon State University, Corvallis. 210 p.
Simard S W and Vyse A 1992 Ecology and management of paper birch and black cottonwood in southern British Columbia. Land Management Report. ISSN 0702-9861; no. 75. B.C. Ministry of Forests, Victoria.
Svejcar T J, Boutton TW and Trent J D 1990 Assessment of carbon allocation with stable carbon isotope labeling. Agron. J. 82, 18- 21.
Van Kessel C, Farrell R E and Pennock D J 1994 Carbon-13 and nitrogen-15 natural abundance in crop residues and soil organic matter. Soil Sci. Soc. Am. J. 58, 382-389.
Van Norman RW and Brown A H 1952 The relative rates of photosynthetic assimilation of isotopic forms of carbon dioxide. Plant Physiol. 27, 691-709.
Wang J R, Simard SW and Kimmins J P 1995 Physiological responses of paper birch to thinning in the ICHmw subzone of British Columbia. For. Ecol. Manage. 73, 177-184.
Wang J R, Zhong A L, Simard S W and Kimmins J P 1996 Aboveground biomass and nutrient accumulation in an age sequence of paper birch (Betula papyrifera) in the Interior Cedar Hemlock zone, British Columbia. For. Ecol. Manage. 83, 27-38.
Waring R H, Schlesinger W H 1985 Forest Ecosystems: Concepts and Management. Academic Press, Inc., Orlando. 340 p.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simard, S.W., Durall, D.M. & Jones, M.D. Carbon allocation and carbon transfer between t Betula papyrifera and t Pseudotsuga menziesii seedlings using a 13C pulse-labeling method. Plant and Soil 191, 41–55 (1997). https://doi.org/10.1023/A:1004205727882
Issue Date:
DOI: https://doi.org/10.1023/A:1004205727882