Abstract
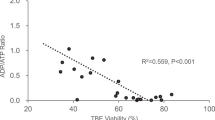
Pig liver is a possible source of hepatocytes for extracorporeal bio-artificial liver devices. In order to evaluate recovered hepatocyte function following enzymatic isolation, we developed a cytochemical method that is based on the capacity of hepatocytes to sequester the anthracycline antitumour drug doxorubicin within intracellular acidic compartments. Doxorubicin is a naturally fluorescent molecule. Thus, the process of drug concentration within hepatocytes can be visualized in living conditions by fluorescence microscopy. Porcine hepatocytes harvested from heart-beating donors were grown either as isolated cell suspensions or as tissue monolayers. Immediately after isolation and at fixed culture times, cells were incubated with 0.1 mM doxorubicin in Hanks' balanced salt solution for 10 min at 37 °C in 5% CO2-humidified atmosphere and observed by fluorescence microscopy. Parallel electron microscopy was performed to compare fluorescence data with general cell morphology. To monitor lysosomal acidification capacity, the fluorescent pH-sensitive vital dye LysoSensor-Blue was used. Doxorubicin fluorescence showed different patterns of nuclear and cytoplasmic staining, according to the time allowed for cell recovery and the culture method. In particular, cytoplasmic fluorescence changed from a diffuse staining, that could be observed after cell isolation and in hepatocyte suspensions, to a punctate perinuclear and pericanalicular fluorescence detectable in fully recovered hepatocyte monolayers. This study indicates that the ‘doxorubicin-fluorescence test’ may be considered a simple and rapid procedure for assessing hepatocyte functional condition. It may provide valuable and ‘real time’ guidelines for judging the correct way these cells are to be collected, preserved and utilized for clinical purposes.
Similar content being viewed by others
References
Altan N, Chen Y, Schindler M, Simon SM (1998) Defective acidification in human breast tumor cells and implications for chemotherapy. J Exp Med 187: 1583–1598.
Ando S, Kamiya K, Yoshimura T, Tsutani H, Ueda T, Uchida M, Nakamura T, Uchino H (1988) DNA-binding characteristics of aclarubicin as compared with daunorubicin and doxorubicin. Anticancer Res 8: 409–415.
Arancia G, Bordi F, Calcabrini A, Diociaiuti M, Molinari A (1995) Ultrastructural and spectroscopic methods in the study of anthracycline-membrane interaction. Pharmacol Res 32: 255–272.
Bachur NR, Moore AL, Bernstein JG, Liu A (1970) Tissue distribution and disposition of daunomycin (NSC-82151) in mice: fluorometric and isotopic methods. Cancer Chemother Rep 54: 89–94.
Bathgate AJ, Hayes PC (1998) Acute liver failure: complications and current management. Hosp Med 59: 195–199.
Berry MN, Edwards AM, Barrit GJ (1991) Assessment of integrity of isolated hepatocytes. In: Berry MN, Edwards AM, Barrit GJ, eds. Isolated Hepatocytes Preparation, Properties and Applications. Amsterdam, New York, Oxford: Elsevier, pp. 83–98.
Bismuth H, Samuel D, Gugenheim J, Castaing D, Bernuau J, Rueff B, Bernhamou JP (1987) Emergency liver transplantation for fulminant hepatitis. Ann Intern Med 107: 337–341.
Coley HM, Amos WB, Twentyman RR, Workman P (1993) Examination by laser scanning confocal fluorescence imaging microscopy of the subcellular localization of anthracyclines in parent and multidrug resistant cell lines. Br J Cancer 67: 1316–1323.
Corno V, Donini A, Lavaroni S, Marcuzzi S, Bigi L, Crivellato E, Pasetto A, Degrassi A, Bresadola F (1997) Rapid and efficient isolation of porcine hepatocytes using an automated cell processor. J Hepatol 26 (Suppl. 1): 108A.
Crivellato E, Candussio L, Rosati AM, Decorti G, Bartoli Klugmann F, Mallardi F (1999) Kinetics of doxorubicin handling in the LLC-PK1 kidney epithelial cell line is mediated by both vesicle formation and P-glycoprotein drug transport. Histochem J 31: 635–643.
Decorti G, Peloso I, Favarin D, Bartoli Klugmann F, Candussio L, Crivellato E, Mallardi F, Baldini L (1998) Handling of doxorubicin by the LLC-PK1 kidney epithelial cell line. J Pharmacol Exp Ther 286: 525–530.
de Lange JH, Schipper NW, Schuurhuis GJ, ten Kate TK, van Heijningen TH, Pinedo HM, Lankelma J, Baak JPA (1992) Quantification by laser scan microscopy of intracellular doxorubicin distribution. Cytometry 13: 571–576.
Diociaiuti M, Molinari A, Calcabrini A, Arancia G (1991) Electron energy-loss spectroscopy analysis of adriamycin-plasma membrane interaction. J Microsc 164: 95–106.
Donini A, Corno V, Lavaroni S, Pellerito R, Bigi L, Pasqualotto A, Belvedere O, Crivellato E, Degrassi A, Bresadola F (1997) Ex vivo preparation of porcine hepatocytes for use in bioartificial hepatic support systems Transplant Proc 29: 1948–1949.
Frezard F, Garnier-Suillerot A (1991) Comparison of the membrane transport of anthracycline derivatives in drug-resistant and drugsensitive K562 cells. Eur J Biochem 196: 483–491.
Harringan PR, Wong KF, Redelmeier TE, Wheeler JJ, Cullis PR (1993) Accumulation of doxorubicin and other lipophilic amines into large unilamellar vesicles in response to transmembrane pH gradient. Biochim Biophys Acta 1149: 329–338.
Hayes JH, Soroka CJ, Rios-Velez L, Boyer JL (1999) Hepatic sequestration and modulation of the canalicular transport of the organic cation, daunorubicin, in the rat. Hepatology 29: 483–493.
Kane SE (1996) Multidrug resistance of cancer cells. Adv Drug Res 28: 181–252.
Lowry OH, Rosenbrough NJ, Fark AL, Randell LJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
Matsumura KN, Guevara GR, Huston H, Hamilton WL, Rikimaru M, Yamasaki G, Matsumura MS (1987) Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery 101: 99–103.
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55: 663–700.
Millot C, Millot JM, Morjani H, Desplaces A, Manfait M (1997) Characterization of acidic vesicles in multidrug-resistant and sensitive cancer cells by acridine orange staining and confocal microspectrofluorimetry. J Histochem Cytochem 45: 1255–1264.
Mustonen P, Kinnunen PK (1993) On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem 268: 1074–1080.
Rabkin SW, Sunga P (1987) The effect of doxorubicin (adriamycin) on cytoplasmic microtubule system in cardiac cells. J Mol Cell Cardiol 19: 1073–1083.
Rozga J, Podesta L, Le Page E, Hoffman A, Morsiani E, Sher L, Woolf GM, Makowka L, Demetriou AA (1993a) Control of cerebral oedema by total hepatectomy and extracorporeal liver support in fulminant hepatic failure. Lancet 342: 898–899.
Rozga J, Williams F, Ro MS, Neuzil DF, Giorgio TD, Backfish G, Moscioni AD, Hakim R, Demetriou AA (1993b) Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology 17: 258–265.
Seidel A, Hasman M, Loeser R, Bunge A, Schefer B, Herzig I, Steidtmann K, Dietel M (1995) Intracellular localization, vesicular accumulation and kinetics of daunorubicin in sensitive and multidrugresistant gastric carcinoma EPG85–257 cells. Virchows Archiv 426: 249–256.
Sussman NL, Chong MG, Koussayer T, He DE, Shang TA, Whisennand HH, Kelly JH (1992) Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology 16: 60–65.
Watanabe FS, Arnout WS, Ting P, Navarro A, Khalili T, Kamohara Y, Kahaku E, Rozga J, Demetriou AA (1999) Artificial liver. Transplant Proc 31: 371–373.
Zunino F, Gambetta R, Di Marco A, Velcich A, Zaccaria A, Quadrifoglio F, Crescenzi V (1977) The interaction of adriamycin and its beta anomer with DNA. Biochim Biophys Acta 476: 38–46.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Crivellato, E., Donini, A., Baccarani, U. et al. Efficiency of Doxorubicin Handling by Isolated Hepatocytes is a Valuable Indicator for Restored Cell Function. Histochem J 32, 535–543 (2000). https://doi.org/10.1023/A:1004198127027
Issue Date:
DOI: https://doi.org/10.1023/A:1004198127027