Abstract
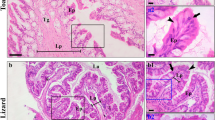
Carbonic anhydrase II (CA II) is present in human oesophageal epithelial cells and probably involved in protecting the mucosa against acidic gastric refluxate. If this is the case, then it is likely that the enzyme will be more concentrated at or near the gastro-oesophageal junction. To answer this question, and determine whether CA II is present and similarly distributed in other species, we also examined the oesophageal epithelium of the rat and pig. In the rat, CA II was largely absent from the oesophageal epithelium, but present in the stratified squamous epithelium of the gastric forestomach as an approximately 2 mm-long collar around the entrance to the corpus, a site that roughly corresponds to the gastro-oesophageal junction in other animals. The enzyme was present mainly in basal and prickle cells. In upper and middle pig oesophagus, CA II was largely confined to basal cells and isolated groups of stratified superficial prickle cells. CA II-containing epithelial cells were highly concentrated in the thickened epithelium at the gastro-oesophageal junction (about four-times thicker than upper or middle). Reactive cells were present throughout the depth of the epithelium, but noticeably more concentrated in the basal and superficial prickle cell layers. CA II was also prominent in the most superficial cell layers in islands of the oesophageal mucosa within the gastric cardia. In man, CA II was confined largely to the basal half of the epithelium in the upper and middle regions of oesophagus. The distribution of CA II at the gastro-oesophageal junction took different forms. In general, there were more CA II-reactive cells at or closer to the lumen. The superficial prickle cell layers tended to exhibit more CA II than the deeper layers, with basal and epibasal cells containing little or no enzyme. In other regions of the same specimens, CA II-containing cells were present from the basal to the most luminal layers. If CA II in oesophageal epithelial cells in the region of the gastro-oesophageal junction (or in the case of the rat the forestomach/corpus junction) is important in the defence against refluxate, then it is in a vulnerable site, since bile salts are potent inhibitors of the enzyme. The action of bile salts on CA II may be an important factor in the initiation of oesophageal disease.
Similar content being viewed by others
References
Argenzio RA (1993) Gastrointestinal motility. In: Swenson MH, Reece WO, eds. Dukes' Physiology of Domestic Animals, 11th Ed. London: Cornell University Press, pp. 336-348.
Argenzio RA, Eiseman J (1996) Mechanisms of acid injury in porcine gastroesophageal mucosa. Am J Vet Res 57: 564-573.
Beuerle JR, Azzazy HM, Styba G, Duh S, Christenson RH (2000) Characteristics of myoglobin, carbonic anhydrase III and the myoglobin/carbonic anhydrase III ratio in trauma, exercise, and myocardial infarction patients. Clin Chim Acta 294: 115-128.
Bivin WS, Crawford MP, Brewer NR (1979) Morphophysiology. In: Baker HJ, Lindsey JR, Weisbroth SH, eds. The Laboratory Rat, Vol 1, Biology and Diseases. New York: Academic Press, pp. 78-79.
Brown CM, Snowdon CF, Slee B, Sandle LN, Rees WDW (1995) Effect of topical oesophageal acidification on human salivary and oesophageal alkali secretion. Gut 36: 649-653.
Cammer W, Zhang H, Cammer M (1993) Glial cell abnormalities in the CNS of the carbonic anhydrase II deficient mutant mouse. J Neurol Sci 118: 1-9.
Christie KN, Thomson C, Morley S, Anderson J, Hopwood D (1995) Carbonic anhydrase is present in human oesophageal epithelium and submucosal glands. Histochem J 27: 587-590.
Christie KN, Thomson C, Xue L, Lucocq JM, Hopwood D (1997) Carbonic anhydrase isoenzymes I, II, III and IV are present in human esophageal epithelium. J Histochem Cytochem 45: 35-40.
Dodgson SJ (1991) Liver mitochondrial carbonic anhydrase (CAV), gluconeogenesis, and ureagenesis in the hepatocyte. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, eds. The Carbonic Anhydrases Cellular Physiology and Molecular Genetics. New York: Plenum Press, pp. 297-306.
Fernley RT (1991) Carbonic anhydrases secreted in saliva. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, eds. The Carbonic Anhydrases Cellular Physiology and Molecular Genetics. New York: Plenum Press, pp. 365-373.
Katada N, Hinder RA, Smyrk TC, Hiki Y, Kakita A (1999) Duodeno-esophageal reflux induces apoptosis in rat esophageal epithelium. Dig Dis Sci 44: 301-310.
Kinoshita M, Kume E, Igarashi S, Saito N, Narita H (1999) Role of salivary mucin in the protection of rat esophageal mucosa from acid and pepsin-induced injury. Am J Physiol-Gastro Liv Physiol 277: G796-G800.
Kivela A, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila A-K, Waheed A, Sly WS, Grubb JH, Shah G, Tureci O, Rajaniemi H (2000) Expression of a novel transmembrane carbonic anhydrase isoenzyme XII in normal human gut and colorectal tumors. Am J Pathol 156: 577-584.
Lang J, Blikslager A, Regina D, Eisemann J, Argenzio R (1998) Synergistic effect of hydrochloric acid and bile acids on the pars esophageal mucosa of the porcine stomach. Am J Vet Res 59: 1170-1176.
Milov DE, Jou WS, Shireman RB, Chun PW (1992) The effect of bile salts on carbonic anhydrase. Hepatology 15: 288-296.
Parkkila S, Parkkila A-K, Juvonen T, Rajaniemi H (1994) Distribution of the carbonic anhydrase isoenzymes I, II and VI in the human alimentary tract. Gut 35: 646-650.
Parkkila S, Parkkila A-K, Lehtola J, Reinilä A, Södervik HJ, Rannisto M, Rajaniemi H (1997) Salivary carbonic anhydrase protects gastroesophageal mucosa from acid injury. Dig Dis Sci 42: 1013-1019.
Robert A (1971) Proposed terminology for the anatomy of the rat stomach. Gastroenterology 60: 344-345.
Roels S, Ducatelle R, Broekaert D (1997) Keratin pattern in hyperkeratotic and ulcerated gastric oesophagea in pigs. Res Vet Sci 62: 165-169.
Salomoni M, Zuccato E, Granelli P, Montorsi W, Doldi SB, Germiniani R, Mussini E (1989) Effect of bile acids on carbonic anhydrase from rat and human gastric mucosa. Scand J Gastroenterol 24: 28-32.
Seto Y, Kobari O (1993) Role of reflux oesophagitis and acid in the development of columnar epithelium in the rat oesophagus. Brit J Surg 80: 467-470.
Smith EM, Calhoun ML (1968) The Microscopic Anatomy of the White Rat: A Photographic Atlas. Ames: Iowa State University Press, pp. 73-77.
Tashian RE (1989) The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays 10: 186-192.
Waheed A, Zhu XL, Sly WS (1992) Membrane-associated carbonic anhydrase from rat lung. Purification characterization, tissue distribution, and comparison with carbonic anhydrase IV' of other animals. J Biol Chem 267: 3308-3311.
Williams D, Thompson DG, Heggie L, Bancewicz J (1993) Responses of the human esophagus to experimental intraluminal distension. Am J Physiol 265: G196-G203.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christie, K.N., Thomson, C. The Distribution of Carbonic Anhydrase II in Human, Pig and Rat Oesophageal Epithelium. Histochem J 32, 753–757 (2000). https://doi.org/10.1023/A:1004105228370
Issue Date:
DOI: https://doi.org/10.1023/A:1004105228370