Abstract
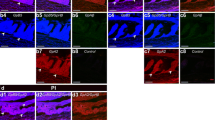
The adenohypophysis of the white seabream (Diplodus sargus) was studied using histochemical and immunocytochemical techniques. The adenohypophysis was composed of rostral pars distalis, proximal pars distalis and pars intermedia. Prolactin (anti-chum salmon prolactin positive) and adrenocorticotropic (anti-human ACTH positive) cells were found in the rostral pars distalis. Prolactin cells were organized into follicles, while ACTH cells were arranged in cords around neurohypophyseal tissue branches that penetrated the rostral pars distalis. In the proximal pars distalis, somatotropic (anti-chum salmon and anti-gilthead seabream growth hormone positive), gonadotropic (anti-chum salmon β-gonadotrophin II and anti-carp β-gonadotrophin II positive, but anti-chum salmon β-gonadotrophin I negative) and thyrotropic (anti-human β-thyrotropin positive) cells were observed. Growth hormone cells were restricted to the dorsal and ventral part of the proximal pars distalis. They were clustered or surrounded the neurohypophyseal branches. Only one type of gonadotrophin cell was identified and they were clustered or isolated in the proximal pars distalis. Scattered groups of thyrotropin cells were located throughout the proximal pars distalis. In the pars intermedia somatolactin (anti-chum salmon and anti-gilthead seabream somatolactin positive) and melanotropic (anti-α-melanotropic hormone positive) cells were localized. In addition, gonadotrophin cells surrounded the pars intermedia or distributed evenly between somatolactin and melanotropic hormone cells. Somatolactin cells were periodic acid-Schiff negative and surrounded the neurohypophyseal branches intermingled with melanotropic cells. These cells were also immunoreactive to anti-human ACTH antiserum.
Similar content being viewed by others
References
Abellan E, Garcia-Alcazar A (1995) Pre-growout and growout experiences with white seabream (Diplodus sargus sargus, Linnaeus, 1758) and sharpsnout seabream (Diplodus puntazzo, Cetti, 1977). Cah Options Mediterr vol. 16. CIHEAM, Zaragoza (Spain).
Abraham M (1971) Ultrastructure of the cell types and the neurosecretory innervation in the pituitary of Mugil cephalus L. from freshwater, the sea, and a hypersaline lagoon. I. The rostral pars distalis. Gen Comp Endocrinol 17: 334-350.
Adams CWM, Sweetenham KV (1958) The hystochemical identification of two types of basophil cell in the normal human adenohypophysis. J Pathol Bacteriol 75: 95-103.
Arias-Garcia AM, Drake Moyano P (1990) Estados juveniles de la ictiofauna en los caños de las salinas de la Bahía deCádiz. Consejo Superior de Investigaciones Científicas, Consejería de Gobernación, Junta de Andalucía. pp. 96-98.
Astola A, Pendón C, Ortiz M, Valdivia MM (1996) Cloning and expression of somatolactin, a pituitary hormone related to growth hormone and prolactin from Gilthead Seabream, Sparus aurata. Gen Comp Endocrinol 104: 330-336.
Baker BI, Wilson JF, Bowley TJ (1984) Changes in pituitary and plasma levels of MSH in teleosts during physiological colour change. Gen Comp Endocrinol 55: 142-149.
Ball JN, Baker BI (1969) The pituitary gland: anatomy and histophysiology. In: Hoar WS, Randall DJ, eds. Fish Physiology New York: Academic Press. vol. 2, pp. 1-205.
Batten TFC (1986) Immunocytochemical demonstration of pituitary cell types in the teleost Poecilia Latipinna, by light and electron microscopy. Gen Comp Endocrinol 63: 139-154.
Batten TFC, Ingleton PM (1987) The hypothalamus and pituitary gland. The structure and function of the hypothalamus and pituitary gland. In: Chester-Jones I, Ingleton PM, Phillips JG, eds. Fundamentals of Comparative Vertebrate Endocrinology New York: Plenum Press, Chap. III, pp. 283-409.
Benjamin M (1978) Cytological changes in prolactin, ACTH and growth hormone cells of the pituitary gland of Pungitius pungitius L. in response to increased environmental salinities. Gen Comp Endocrinol 36: 48-58.
Bern HA (1983) Functional evolution of prolactin and growth hormone in lower vertebrates. Amer Zool 23: 663-671.
Bjornsson, B Th (1997) The biology of salmon growth hormone: from daylight to dominance. Fish Physiol Biochem 17: 9-24.
Cambré ML, Verdonck W, Ollevier F, Vandesande F, Batten TFC, Kühn ER (1986) Immunocytochemical identification and localization of the different cell types in the pituitary of the seabass (Dicentrarchus labrax). Gen Comp Endocrinol 61: 368-375.
Cavari B, Noso T, Kawauchi H (1995) Isolation and characterisation of somatolactin from pituitary glands of gilthead sea bream Sparus aurata. Aquaculture 137: 171-178.
Cejas J, Samper M, Jerez S, Flores R, Villamandos J (1993) Culture perspectives of common pandora (Pagellus erythrinus) and white sea bream (Diplodus sargus); Preliminary growth results in comparison with red sea bream (Sparus aurata). In: Cervino A, Landin A, De Coo A, Guerra A, Torre M, eds. Actas de IV Congreso Nacional de Acuicultura Centro de Investigaciones Marinas de Pontevedra, Spain, pp. 127-132.
Copeland PA, Thomas P (1993) Isolation of gonadotropin subunits and evidence for two distinct gonadotropins in Atlantic croacker (Micropogonias undulatus). Gen Comp Endocrinol 91: 115-125.
Divanach P, Kentouri M, Paris J (1982) Etapes du d´eveloppement ambryonnaire et larvaire du sar, Diplodus sargus L., en elevage. Aquaculture 27: 339-353.
Dores RM (1990) The proopiomelanocortin family. In: Epple A, Scanes CG, Stetson, MH (eds.), Progress in comparative endocrinology, New York: Wiley-Liss, pp. 22-27.
Dubourg P, Burzawa-Gerard E, Chambolle P, Kah O (1985) Light and electron microscopic identification of gonadotrophic cells in the pituitary of the goldfish by means of immunocytochemistry. Gen Comp Endocrinol 59: 472-481.
Elizur A, Zmora N, Rosenfeld H, Meiri I, Hassin S, Gordin H, Zohar Y (1996) Gonadotropins beta-GtH I and beta-GtH II from the gilthead seabream, Sparus aurata. Gen Comp Endocrinol 102: 39-46.
Farbridge KJ, Leatherland JF (1986) Acomparative immunohystochemical study of the pars distalis in six species of teleost fishes. Fish Physiol Biochem 1: 63-74.
Farmer SW, Papkoff H (1979) Comparative biochemistry of pituitary growth hormone, prolactin and the glycoprotein hormones. In: Barrington B, ed. Hormones and evolution New York: Academic Press, pp. 525-529.
Follénius E, Dubois MP (1980) Localization of anti-ACTH, anti-MSH, and anti-α-endorphin reactive sites in the fish pituitary. In: Jutisz M, Mckerns KW eds. Synthesis and release of adenohypophyseal hormones, Plenum Publishing Corporation, pp. 197-208.
Follénius E, Doerr-Schott J, Dubois M P. (1978) Immunocytology of pituitary cells from teleost fishes. Int Rev Cytol 54: 193-233.
García-Hernández MP, García-Ayala A, Elbal MT, Agulleiro B (1996) The adenohypophysis of Mediterranean yellowtail, Seriola dumerilii (Risso, 1810): an immunocytochemical study. Tissue Cell 28 (5): 577-585.
Gen K, Okuzawa K, Senthilkumaran B, Tanaka H, Moriyama S, Kagawa H (2000) Unique expression of gonadotropin-I and-II subunit genes in male and female red seabream (Pagrus major) during sexual maturation. Biol Reprod 63: 308-319.
Henderson IW, Garland HO (1980) The interrenal gland in pisces: Part 2. Physiology. In: Chester-Jones I, Henderson IW, eds. General, Comparative and Clinical Endocrinology of the Adrenal Cortex. New York: Academic Press, vol. 3, pp. 473-523.
Hirano T (ed.) (1986) The spectrum of prolactin actions in teleosts. Comparative Endocrinology: Developments and Directions. C.L. Ralph, ed. pp. 53-74. A.R. Liss.
Holmes RL, Ball JN (eds.) (1974) The pituitary gland: a comparative account, London: Cambridge University Press, pp. 170-220.
Huang L, Specker JL (1994) Growth hormone-and prolactin-producing cells in the pituitary gland of striped bass (Morone saxatilis): Immunocytochemical characterization at different life stages. Gen Comp Endocrinol 94: 225-236.
Iturriza FC, Estivariz FE (1986) Lack of glycosilation of proopiomelanocortin might account for periodic acid-Schiff-negative reaction in ACTH cells of teleost fishes. Gen Comp Endocrinol 61: 229-236.
Kaneko T (1996) Cell biology of somatolactin. Int Rev Cytol 169: 1-24.
Kaneko T, Kakizawa S, Yada T, Hirano T (1993) Gene expression and intracellular localization of somatolactin in the pituitary of rainbow trout. Cell Tissue Res 272: 11-16.
Kawauchi H, Yasuda A (1989) Evolutionary aspects of growth hormones from non-mammalian species. In: Muller EE, Cocchi D, Locatelli V, eds. Advances in growth hormone and growth factor research Berlin and Heidelberg: Springer-Verlag, pp. 51-68.
Kawauchi H, Abe KI, Takahashi A, Hirano T, Hasegawa S, Naito N, Nakai Y (1983) Isolation and properties of chum salmon prolactin. Gen Comp Endocrinol 49: 446-458.
Kawauchi H, Moriyama S, Yasuda A, Yamaguchi K, Shirahata K, Kato, J, Hirano T (1986) Isolation and characterisation of chum salmon growth hormone. Arch Biochem Biophys 244: 542-552.
Magliulo-Cepriano L, Schreibman MP, Blüm, V (1994) Distribution of variant forms of immunoreactive gonadotropin-releasing hormone and ß-gonadotropins I and II in the platyfish, Xiphophorus maculatus, from birth to sexual maturity. Gen Comp Endocrinol 94: 135-150.
Mancera JM, Mccormick SD (1998) Osmoregulatory actions of the GH/IGF axis in non-salmonids teleosts. Comp Biochem Physiol 121 B, 43-48.
Mancera JM, Fernández-Llebrez P, Grondona JM, Pérez-Fígares JM (1993) Influence of environmental salinity on prolactin and corticotropic cells in the gilthead seabream, Sparus aurata. Gen Comp Endocrinol 90: 220-231.
Mancera JM, Fernández-Llebrez P, Pérez-Figares JM (1995) Effects of decreased environmental salinity on growth hormone cells in the gilthead sea bream (Sparus aurata). J Fish Biol 46: 494-500.
Martínez-Barberá JP, Pendón C, Rodríguez RB, Pérez-Sánchez J, Valdivia MM (1994) Cloning expression and characterization of a recombinant gilthead seabream, Sparus aurata. Gen Comp Endocrinol 96: 179-188.
Mazzola A, Rallo B, Giliberto S, Ceccarelli E (1983) Report on artificial reproduction and first stage of the breeding of the sargo (Diplodus sargus L.). In: Stickney RR, Mayrs SP (eds.),Warm water fish culture. World Maric Soc Spec Publ 3: pp.124-132.
Mclean E, Donaldson EM (1993) The role of growth hormone in growth of poikilotherms. In: Schreibman MP, Scanes CG, Pang PKT, eds. The Endocrinology of Growth, Development and Metabolism in Vertebrates. New York: Academic Press, pp. 43-71.
Mcmanus JFA (1948) Histological and histochemical uses of periodic acid. Stain Technol 23: 99-108.
Micale V, Perdichizzi F, Santangelo G (1987) The gonadal cycle of captive white bream, Diplodus sargus (L.). J Fish Biol. 31: 435-440.
Mordenti O, Roncarati A, Melotti P, Gennari L, Dees A (1996) Breeding and feeding of juveniles of the white seabream (Diplodus sargus L.). Biol Mar Mediterr 1: 425-426.
Munro AD (1985) The structure of the adenohypophysis of Aequidens pulcher (Teleostei, Cichlidae). I. Histological and immunohystochemical studies. Gen Comp Endocrinol 60: 215-226.
Nagahama Y, Olivereau M, Farmer SW, Nishioka RS, Bern HA (1981) Immunocytochemical identification of the prolactin-and growth hormone-secreting cells in the teleost pituitary with antisera to tilapia prolactin and growth hormone. Gen Comp Endocrinol 44: 389-395.
Naito N, Hyodo S, Okumoto N, Urano A, Nakai Y (1991) Differential production and regulation of gonadotropins (GTH I and GTH II) in the pituitary gland of rainbow trout Oncorhynchus mykiss, during ovarian development. Cell Tissue Res 266: 457-467.
Nozaki M, Naito N, Swanson P, Miyata K, Nakai Y, Oota Y, Suzuki, K, Kawauchi H (1990) Salmonid pituitary gonadotrophs I. Distinct cellular distributions of two gonadotropins, GTH I and GTH II. Gen Comp Endocrinol 77: 348-357.
Okada T, Kawazoe I, Kimura S, Sasamoto Y, Aida K, Kawauchi H (1994) Purification and characterization of gonadotropin I and II from pituitary glands of tuna (Thunnus obesus). Int J Peptide Protein Res 43: 69-80.
Olivereau M, Nagahama Y (1983) Immunocytochemistry of gonadotropic cells in the pituitary of some teleost species. Gen Comp Endocrinol 50: 252-260.
Olivereau M, Rand-Weaver M (1994) Immunocytochemical study of the somatolactin cells in the pituitary of pacific salmon, Oncorhynchus nerka, and O. keta at some stages of the reproductive cycle. Gen Comp Endocrinol 93: 28-35.
Parhar YS, Nagahama Y, Grau EG, Ross RM (1998) Immunocytochemical and ultrastructural identification of pituitary cell types in the protogynous Thalassoma duperrey during adult sexual ontogeny. Zool Sci 15: 263-276.
Pendón C, Martínez-Barberá JP, Ortiz M, Valdivia MM (1996) Bacterial production and purification of the fish pituitary hormone somatolactin. Prot Expr Purif 7: 389-394.
Pierce JG, Parsons TF (1981) Glycoprotein hormones: structure and function. Annu Rev Biochem 50: 465-495.
Quesada J, Lozano MT, Ortega A, Agulleiro B (1988) Immunocytochemical and ultrastructural characterisation of the cell types in the adenohypophysis of Sparus aurata L. (Teleost). Gen Comp Endocrinol 72: 209-225.
Rand-Weaver M, Kawauchi H (1993) Growth hormone, prolactin and somatolactin: a structural overview. In: Hochachka PW, Mommsen TP, eds. Biochemistry and molecular biology of fishes. Amsterdam: Elsevier, vol. 2, pp. 39-56.
Rand-Weaver M, Baker BJ, Kawauchi H (1991) Cellular localization of somatolactin in the pars intermedia of some teleost fishes. Cell Tissue Res 263: 207-215.
Rendón C, Rodríguez-Gómez FJ, Muñoz-Cueto JA, Piñuela C, Sarasquete C (1997) An immunocytochemical study of pituitary cells of the Senegalese sole, Solea senegalensis (Kaup 1858). Histochem J 29: 813-822.
Sakamoto T, Mccormick SD, Hirano T (1993) Osmoregulatory actions of growth hormone and its mode of action in salmonids: a review. Fish Physiol Biochem. 11: 155-164.
Sarasquete C, Muñoz-Cueto JA, García-García A, Gonzalez De Canales, ML, Rodríguez FJ, Piñuela C, Rodríguez RB (1997) Histochemical and immunocytochemical study of gonadotropic pituitary cells of the killi-fish, Fundulus heteroclitus, during annual reproductive cycle. Scientia Marina 61 (14): 439-499.
Schreibman MP, Margolis-Kazan H (1979) The immunocytochemical localization of gonadotropin, its subunits and thyrotropin in the teleost Xiphophorus maculatus. Gen Comp Endocrinol 39: 467-474.
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1968) The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antiperoxidase) and its use in identification of spirochetes. J Histochem Cytochem 18: 315-333.
Suzuki K, Kawauchi H, Nagahama Y(1988a) Isolation and characterization of two distinct gonadotropins from chum salmon pituitary glands. Gen Comp Endocrinol 71: 292-301.
Suzuki K, Kawauchi H, Nagahama Y (1988b) Isolation and characterization of subunits of two distinct gonadotropins from chum salmon pituitary glands. Gen Comp Endocrinol 71: 302-306.
Swanson P, Suzuki K, Kawauchi H, Dickhoff WC (1991) Isolation and characterization of two coho salmon gonadotropins, GTH I and GTH II. Biol Reprod 44: 29-38.
Tanaka M, Tanangonan JB, Tagawa M, De Jesús EG, Nishida H, Isaka M, Kimura R, Hirano T (1995) Development of the pituitary, thyroid and interrenal glands and applications of endocrinology to the improved rearing of marine fish larvae. Aquaculture, 135: 111-126.
Toubeau G, Poilve A, Baras E, Nonclercq D, De Moor S, Beckers JF, Dessy-Doise C, Heuson-Stiennon JA (1991) Immunocytochemical study of cell type distribution in the pituitary of Barbus barbus (Teleostei, Cyprinidae). Gen Comp Endocrinol 83: 35-47.
Ueda H, Young G, Nagahama Y (1983) Immunocytochemical identi-fication of thyrotropin (TSH)-producing cells in pituitary glands of several species of teleosts with antiserum to human TSH ß subunit. Cell Tissue Res 231: 199-204.
Van Putten LJA, Van Oordt PGWJ, Terlou M, Peute J (1983) Histophysiological and immunocytochemical study on the nature of the thyrotrops in the pituitary of the immature rainbow trout, Salmo gairdneri. Cell Tissue Res 231: 185-198.
Van Zoest ID, Heijmen PS, Cruijsen PMJM, Jenks BG (1989) Dynamics of background adaptation in Xenopus laevis: Role of catecholamines and melanophore stimulating hormone. Gen Comp Endocrinol 76: 19-28.
Villaplana M, García-Ayala A, García-Hernández MP, Agulleiro B (1997) Ontogeny of immunoreactive somatolactin cells in the pituitary of gilthead sea bream (Sparus aurata L., Teleostei). Anat Embryol 196: 227-234.
Vissio PG, Somoza GM, Maggese MC, Paz DA, Strüsmann CA (1997) Structure and cell type distribution in the pituitary gland of pejerrey Odontesthes bonariensis. Fisheries Science 63(1): 64-68.
Wendelaar-Bonga SE (1997) The stress response in fish. Physiol Rev 77: 591-625.
Yan HY, Thomas P (1991) Histochemical and immunocytochemical identification of the pituitary cell types in three sciaenid fishes: Atlantic croaker (Micropogonias undulatus), spotted seatrout (Cynoscion nebulosus), and red drum (Sciaenops ocellatus). Gen Comp Endocrinol 84: 389-400.
Yoshiura Y, Sohn YC, Munakata A, Kobayashi M, Aida K (1999) Molecular cloning of the cDNA encoding the beta subunit of thyrotropin and regulation of its gene expression by thyroid hormones in the goldfish, Carassius auratus. Fish Physiol Biochem 21: 201-210.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Segura-Noguera, M., Laíz-Carrión, R., Martín del Río, M. et al. An Immunocytochemical Study of the Pituitary Gland of the White Seabream (Diplodus Sargus). Histochem J 32, 733–742 (2000). https://doi.org/10.1023/A:1004101127461
Issue Date:
DOI: https://doi.org/10.1023/A:1004101127461