Abstract
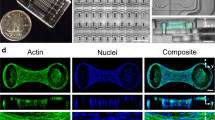
This study reports the cytoskeletal organisation within chondrocytes, isolated from the superficial and deep zones of articular cartilage and seeded into agarose constructs. At day 0, marked organisation of actin microfilaments was not observed in cells from both zones. Partial or clearly organised microtubules and vimentin intermediate filaments cytoskeletal components were present, however, in a proportion of cells. Staining for microtubules and vimentin intermediate filaments was less marked after 1 day in culture however than on initial seeding. For all three cytoskeletal components there was a dramatic increase in organisation between days 3 and 14 and, in general, organisation was greater within deep zone cells. Clear organisation for actin microfilaments was characterised by a cortical network and punctate staining around the periphery of the cell, while microtubules and vimentin intermediate filaments formed an extensive fibrous network. Cytoskeletal organisation within chondrocytes in agarose appears, therefore, to be broadly similar to that described in situ. Variations in the organisation of actin microfilaments between chondrocytes cultured in agarose and in monolayer are consistent with a role in phenotypic modulation. Vimentin intermediate filaments and microtubules form a link between the plasma membrane and the nucleus and may play a role in the mechanotransduction process.
Similar content being viewed by others
References cited
Aydelotte MB, Kuettner KE (1988) Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix products. Conn Tiss Res 18: 205-222.
Aydelotte MB, Greenhill RR, Kuettner KE (1988) Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Conn Tiss Res 18: 223-234.
Bayliss MT, Venn M, Maroudas A, Ali SY (1983) Structure of proteoglycans from different layers of human articular cartilage. Biochem J 209: 387-400.
Benjamin M, Archer CW, Ralphs JR (1994) Cytoskeleton of cartilage cells. Micro Res Tech 28: 372-377.
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes re-express the differentiated collagen phenotype when cultured in agarose gels. Cell 30: 215-224.
Benya PD, Brown PD, Padilla SR (1988) Microfilament modification by dihydrocytochalasin B causes retinoic acid-modified chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Biol Chem 106: 161-170.
Benya PD, Padilla S, Nimni ME (1978) Independent regulation of collagen types of chondrocytes during the loss of differentiated function in culture. Cell 15: 1313-1321.
Brown PD, Benya PD (1988) Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin B-induced reexpression. J Cell Biol 106: 171-179.
Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB (1995) Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Science 108: 1497-1508.
Chang J, Poole CA (1997) Confocal analysis of the molecular heterogeneity in the pericellular microenvironment produced by adult canine chondrocytes cultured in agarose gel. Histochem J 29: 515-528.
Durrant LA, Archer CW, Benjamin M, Ralphs JR (1999) Articular chondrocytes reorganise their cytoskeleton in response to changing mechanical conditions in organ culture. J Anat 194: 343-353.
Edwards CA, O'Brien WD (1980) Modified assay for determination of hydroxyproline in a tissue hydrolysate. Clinica Chimica Acta 104: 161-167.
Eggli PS, Hunziker EB, Schenk RK (1988) Quantitation of structural features characterizing weight and less-weight bearing regions in articular cartilage:Astereological analysis of medial femoral condyles in young adult rabbits. Anat Rec 222: 217-227.
Farmer SR, Dike LE (1989) Cell shape and growth control: Role of cytoskeleton-extracellular matrix. In: Stein WD, Bonner F, eds. Cell Shape: Determinants, Regulation and Regulatory Role. San Diego: Academic Press. pp. 173-204.
Farndale RW, Sayers CA, Barrett AJ (1982) A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Conn Tiss Res 9: 247-248.
Freeman PM, Natarajan RN, Kimura JH, Andriacchi TP (1994) Chondrocyte cells respond mechanically to compressive loads. J Orthop Res 12: 311-320.
Geiger B, Singer SJ (1980) Association of microtubules and intermediate filaments in chicken gizzard cells as detected by double immunofluorescence. Proc Natl Acad Sci USA 77: 4769-4773.
Ghadially FN (1983). Articular cartilage. In: Ghadially FN, ed. The Fine Structure of Synovial Joints. London: Butterworths & Co Ltd. pp. 42-79.
Guilak F (1995) Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech 28: 1529-1541.
Ingber D, Dike L, Hansen L, Karp S, Liley H, Maniotis A, Mcnamee H, Mooney D, Plopper G, Sims J, Wang N (1994) Cellular tensegrity: Exploring how mechanical changes in the cytoskeleton regulate cell growth, migration and tissue pattern during morphogenesis. Internat Rev Cytol 150: 173-224.
Janmey PA (1998) The cytoskeleton and cell signalling: component localization and mechanical coupling. Physiol Rev 78: 763-781.
Knight MM, Lee DA, Bader DL (1996) Distribution of chondrocyte deformation in compressed agarose gel using confocal microscopy. Cell Eng 1: 97-102.
Knight MM, Lee DA, Bader DL (1998) The influence of elaborated pericellular matrix on the deformation of isolated chondrocytes cultured in agarose. Biochem Biophys Acta 1405: 67-77.
Lee DA, Bader DL (1995) The development and characterisation of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In vitro Cell Dev Biol 13: 828-835.
Lee DA, Bader DL (1997) Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res 15: 181-188.
Lee DA, Noguchi T, Knight MM, O'Donnell L, Bentley G, Bader DL (1998) The response of chondrocyte sub-populations cultured within unloaded and loaded agarose. J Orthop Res 16: 726-733.
Linss W, Neopert G, Langbein L (1986) Intermediate filaments in cells of hyaline cartilage. Acta Histochem 80: 29-34.
Loty S, Forest N, Boulekbache H, Sautier J (1995) Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesis: A morphological study. Biol Cell 83: 149-161.
Mallein-Gerin F, Garrone R, Van Der Rest M (1991) Proteoglycan and collagen synthesis are correlated with actin organisation in dedifferentiating chondrocytes. Eur J Cell Biol 56: 364-373.
Maniotis AJ, Chen CS, Ingber DE (1997) Demonstration of mechanical connections between integrins, cytoskeletal filaments and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94: 849-854.
Marchisio PC, Capasso O, Nitsch L, Cancedda R, Gionti E (1984) Cytoskeleton and adhesion patterns of cultured chick embryo chondrocytes during cell spreading and Rous sarcoma virus transformation. Exp Cell Res 151: 332-343.
Marles PJ, Hoyland JA, Parkinson R, Freemont AJ (1991) Demonstration of variation in chondrocyte activity in different zones of articular cartilage: an assessment of the value of in-situ hybridization. Int J Exp Pathol 72: 171-182.
Mayne R, Vail MS, Mayne PM, Miller EJ (1976) Changes in the type of collagen synthesised as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci USA 73: 1674-1678.
Meachim G, Stockwell RA (1979) The matrix. In: ed. Freeman MAR Adult Articular Cartilage. London: Pitman Medical. pp. 1-67.
Newman P, Watt FM (1988) Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Exp Cell Res 178: 192-210.
Palfrey AJ, Davies DV (1966) The fine structure of chondrocytes. J Anat 100: 213-226.
Parkkinen JJ, Lammi MJ, Inkinen R, Jortikka M, Tammi M, Virtanen I, Helminen HJ (1995) Influence of short term hydrostatic pressure on organisation of stress fibres in cultured chondrocytes. J Orthop Res 13: 495-502.
Paukkonen K, Helminen HJ (1987a) Rough endoplasmic reticulum and fine intracytoplasmic filaments in articular cartilage chondrocytes of young rabbits: a stereological morphometric study using transmission electron microscopy. J Anat 152: 47-54.
Paukkonen K, Helminen HJ (1987b) Chondrocyte ultrastructure in exercise and experimental osteoarthrosis. A stereologic morphometric study of articular cartilage of young rabbits using transmission electron microscopy. Clin Orthop Rel Res 224: 284-288.
Schliwa M, Van Blerkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 90: 225-235.
Stockwell RA, Meachim G (1979) The chondrocytes. In: Freeman MAR, ed. Adult Articular Cartilage. London: Pitman Medical. pp. 69-144.
Takigawa M, Takano T, Shirai E, Suzuki F (1984) Cytoskeleton and differentiation: effects of cytochalasin B and colchicine on expression of the differentiated phenotype of rabbit costal chondrocytes in culture. Cell Diff 14: 197-204.
Traub P, Shoeman RL (1994) Intermediate filament proteins: cytoskeletal elements with gene regulatory function? Int Rev Cytol 154: 1-103.
Von der Mark, K, Conrad G (1979) Cartilage cell differentiation. Clin Orthop Rel Res 139: 185-205.
Walter I, Egerbacher M, Wolfesberger B, Seiberl G (1998) Confocal laser scanning microscopy of chondrocytes in vitro: cytoskeletal changes after quinolone treatment. Scanning 20: 511-515.
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124-1127.
Watt FM, Dudhia J (1988) Prolonged expression of differentiated phenotype by chondrocytes cultured at low density on a composite substrate of collagen and agarose that restricts cell spreading. Differentiation 38: 140-147.
Weiss C, Rosenberg L, Helfet AJ (1968). An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg 50A: 663-674.
Wong M, Wuethrich P, Eggli P, Hunziker EB (1996) Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res 14: 424-432.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Idowu, B.D., Knight, M.M., Bader, D.L. et al. Confocal Analysis of Cytoskeletal Organisation within Isolated Chondrocyte Sub-populations Cultured in Agarose. Histochem J 32, 165–174 (2000). https://doi.org/10.1023/A:1004095207330
Issue Date:
DOI: https://doi.org/10.1023/A:1004095207330