Abstract
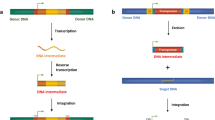
Given that transposons are so abundant in mammalian genomes, it is natural to assume that through their maintenance the host gains some net benefit. This need not be true; sexual reproduction allows a transposon to go to fixation if the reduction in fitness of the host is anything less than two-fold. Obligate outcrossing sexual reproduction therefore favors the evolution of aggressive transposons, which in turn select for the evolution of host mechanisms that suppress transposon activity. Hosts that have asexual or self-fertilizing generations will select for transposons that are more benign and self-limiting than those of obligate sexuals, and obligate asexuals and uniparental organelle genomes will be free of active transposons if these impose any fitness penalty. We are interested in host mechanisms that suppress transposons in sexuals and have found that mammals (all of which are obligate sexuals) control their large populations of potentially active retroposons by methylating the five position of cytosine residues within promoter elements. This causes strong transcriptional repression and assembly of the affected sequences into the condensed state. Methylation also causes permanent inactivation in the germline by driving C→T transition mutations at methylated sites. It is now known that methylation remains in place for the large majority of the life of germ cells and is essential for control of the very large transposon burden. There is pressure on transposons to evolve mechanisms that overcome host suppression, and over evolutionary time, the balance swings back and forth between parasite and host. The ability of the mammalian genome to absorb and accumulate additional transposons has caused the amount of reverse transcriptase coding sequence in the human genome to far exceed the sum total of all cellular coding sequence. While transposons could, in principle, contribute functions useful to the host, the fact that asexual species and uniparental organelle genomes lack transposons is strong evidence that transposons have a net deleterious effect even in genomes that might be thought to require an additional source of plasticity. The abundance of transposons in many genomes cannot be taken as evidence of a mutualistic relationship, and the conflict between transposons and genomes may have actually retarded rather than accelerated evolution. It is suggested that the relationship between sex and transposons is as follows: (i) Obligate sexuals will tend to harbor aggressive transposons limited largely by host suppressive mechanisms, which in mammals involve methylation of transposon promoters. (ii) The aggressiveness of transposons in facultative sexuals and self-fertilizing sexuals will be in part self-limited and will be proportional to the relative frequency of asexual and outcrossing sexual generations. (iii) Obligate asexuals and organelles transmitted in a uniparental manner will have no active transposons if these have a net negative effect on host fitness.
Similar content being viewed by others
References
Bestor, T.H., 1990. DNA methylation: How a bacterial immune function has evolved into a regulator of gene expression and genome structure in higher eukaryotes. Phil. Trans. Royal Soc. Lond. B 326: 179–187.
Bestor, T.H., 1997. The host defense function of genomic methylation patterns, pp. 480–486 in Epigenetics, edited by A.P. Wolffe. Wiley, Chichester (Novartis Foundation Symposium) 18.
Bestor, T.H. & A. Coxon, 1993. The pros and cons of DNA methylation. Curr. Biol. 3: 384–386.
Bestor, T.H. & Tycko, B.: 1996. Creation of genomic methylation patterns. Nat. Genet. 12: 363–367.
Bird, A.P.: 1995. Gene number, noise reduction and biological complexity. Trends Genet. 11: 94–100.
Bird, A., 1997. Does DNA methylation control transposition of selfish elements in the germline? Trends Genet. 13: 469–472.
Capy, P., C. Bazin, D. Higuet & T. Langin, 1998. Dynamics and evolution of transposable elements. Springer-Verlag, Berlin.
Cooper, D.N. & H. Youssoufian, 1988. The CpG dinucleotide and human genetic disease. Hum. Genet. 178: 151–155.
Danilevskaya, O.N., I.R. Arkhipova, K.L. Traverse & M.L. Pardue, 1997. Promoting in tandem: the promoter for telomere transposon HeT-A and implications for the evolution of retroviral LTRs. Cell 88: 647–655.
Darwin, D., 1871. The Descent of Man. John Murray, London.
DeBerardinis, R.J., J.L. Goodier, E.M. Ostertag & H.H. Kazazian, 1998. Rapid amplification of a retrotransposon subfamily is evolving the mouse genome. Nat. Genet. 20: 288–290.
Doolittle, W.F. & C. Sapienza, 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603.
Kobayashi, I., 1998. Selfishness and death: raison d'etre of restriction, recombination and mitochondria. Trends Genet. 14: 368–374.
Hickey, D.A., 1982. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics 101: 519–531.
Partridge, L. & L.D. Hurst, 1998. Sex and conflict. Science 281: 2003–2008.
Manes, C. & P. Menzel, 1981. Demethylation of CpG sites in DNA of early rabbit trophoblast. Nature 293: 589–590.
Mertineit, C., J.A. Yoder, T. Taketo, D.W. Laird, J.M. Trasler & T.H. Bestor, 1998. Sex-specific exons control DNA methyltransferase in mammalian germ cells. Development 125: 889–897.
Orgel, L.E. & F.H. Crick, 1980. Selfish DNA: the ultimate parasite. Nature 284: 604–607.
Simmen, M.W., S. Leitgeb, J. Charlton, S.J. Jones, B.R. Harris, V.H. Clark & A. Bird, 1999. Nonmethylated transposable elements and methylated genes in a chordate genome. Science 283: 1164–1167.
Selker, E.U., 1997. Epigenetic phenomena in filamentous fungi: useful paradigms or repeat-induced confusion? Trends Genet. 13: 296–301.
Smit, A.F., 1996. The origin of interspersed repeats in the human genome. Curr. Opin. Genet. Dev. 6: 743–748.
Smit, A.F., 1999. Interspersed repeats and other momentos of transposable elements in mammalian genomes. Curr. Opin. Genet. Devel. 9: 657–663.
Tabara, H., M. Sarkissian, W.G. Kelly, J. Fleenor, A. Grishok, L. Timmons, A. Fire & C.C. Mello, 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132.
Walsh, C.P. & T.H. Bestor, 1999. Cytosine methylation and mammalian development. Genes Dev. 13: 26–34.
Walsh, C.P., J.R. Chaillet & T.H. Bestor, 1998. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 20: 116–117.
Yoder, J.A., C.P. Walsh & T.H. Bestor, 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 13: 335–340.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bestor, T.H. Sex brings transposons and genomes into conflict. Genetica 107, 289–295 (1999). https://doi.org/10.1023/A:1003990818251
Issue Date:
DOI: https://doi.org/10.1023/A:1003990818251