Abstract
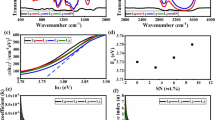
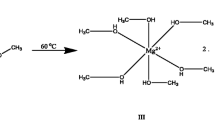
The efficiency of 2-nitrophenylpyruvic acid as a cathode material in a magnesium/zinc based primary battery is examined. The discharge performance of the cell is investigated under different parametric variations such as temperature, nature of electrolyte, current drain and zeolite modification. A 7e reduction seems to be responsible for the electrochemical reaction causing the reduction of 2-nitrophenylpyruvic acid to an indole intermediate which is oxidatively cleaved to form anthranilic acid as the end-of-discharge product. Participation of oxygen in the reduction process is indicated. The discharge capacity is 1.03 Ah g−1, the highest value ever observed in organic batteries.
Similar content being viewed by others
References
R. Glicksman and C.K. Morehouse, J. Electrochem. Soc. 105 (1958) 295.
A. Sivashanmugam, G. Kumar and N. Muniyandi, Ext. Abstr. Proc. Meet. The Electrochem. Soc., Honolulu HI, USA (16-21 May 1993), Abstr. 101, p. 151.
K. Sivasamy, S. Rajeswari and K. Dakshinamurthi, J. Power Sources 25 (1989) 295.
G. Kumar, A. Sivahanmugam and N. Muniyandi, J. Power Sources 39 (1992) 121.
A.L. Endrey and T.A. Reilly, Proceedings of the 22nd Annual Power Sources Conference (May 1989), p. 51.
R. Thirunakaran, S. Vasudevan, A. Sivashanmugam, G. Kumar and N. Muniyandi, J. Power Sources 58 (1996) 213.
G. Kumar, A. Sivashanmugam and R. Sridharan, J. Electrochem. Soc. 140 (1993) 3087.
G. Kumar, A. Sivashanmugam and N. Muniyandi, J. Appl Electrochem. 23 (1993) 265.
R. Renuka, J. Power Sources, 1999, in press.
R. Renuka, S. Saravanan and P.C. Srinivasan, Proceedings of the 6th Intemational Symposium on ‘Advances in Electrochemical Science &Technology’, SEAST (26-28 Nov. 1998), Chennai, India, in press.
Van der Lee, Rec. Trev. Chem. 44 (1925) 1089.
J.P. Harivel, J. Doll, R.S. Cattle and R.J. Gale, in D.H. Collins (Ed.), Power Sources, 4, Oriel Press, Newcastle upon Tyne, pp. 51-61.
Furukawa Battery Co., Ltd., Jpn. Patent 83 122 63 (1983); Chem. Abstr. 99 (1983) 25450g.
T. Inoue, K. Kobayashi and K. Matsuo, Jpn. Patent 87 193 060 (1987); Chem. Abstr. 108 (1988) 8802g.
A. Moor, Ger. Patent 1 302 003 (1969); Chem. Abstr. 73 (1970) 126473g.
M. Crucena, E. Popovice and A. Vasile, Rom. Patent RO 78 511 (1982); Chem. Abstr. 99 (1983) 125685c.
K. Miwa, K. Iwayama and H. Fukui, Jpn. Patent 87 241 265 (1987); Chem. Abstr. 108 (1988) 24622d.
Debra R. Rolison, Chem. Rev. 90 (1990) 867.
D.R. Rolison, C.A. Bessel, M.D. Baker, C. Seneratne and J. Zhang, J. Phys. Chem. 100 (1996) 8610.
T. Bein and P. Enzel, Angew. Chem. Int. Ed. (Engl) 28 (1989) 1692.
T. Bein and P. Enzel, Synth. Metals 29 (1989) El63; J. Phys. Chem 93 (1989) 6270.
A.J. Bard and T.E. Mallou, in ‘Molecular Design of Electrode Surfaces’, edited by R.W. Murray, cited in [15].
C. Johansson, L. Risinger, L. Fallth and L. Huy, Sci. Inst. 49 (1980) 47.
R.P. Townsend, Pure Appl. Chem. 58 (1986) 1359.
J. Sarradin, J. Louvet, R. Mersina and J. Perchon, Zeolites 4 (1984) 157.
D.W. Breck, ‘Zeolite Molecular Sieves: Structure, Chemistry & Use’ (Wiley-Interscience, New York, 1974).
W.M. Meier and D.H. Olsen, ‘Atlas of Zeolite Structure Types’ (Juris Press, Zurich, 1978).
J.A. Jacobs, ‘Carboniogenic Activity of Zeolites’ (Elsevier, Amsterdam, 1977).
J.B. Peri, in ‘Catalysis: Science and Technology’ edited by J.R. Anderson and R.M. Boudart (Springer-Verlag, Berlin, 1984), p. 191.
W.F. Holderich and H. Van Bekkum, Stud. Surf. Sci. Catal. 58 (1991) 631.
V. Ganesan and R. Ramaraj, Langmuir 14 (1998) 2497.
K. Pitchumani, Joy Abraham, Prevost Nicolettle and V. Ramamurthy, Chem. Commun. (1997) 127.
R. Renuka and D. Kalaiselvi, J. Chem. Technol. Biotechnol, 1999, in press.
R. Renuka and D. Kalaiselvi, J. Appl. Electrochem. 29 (1999) 797.
J. Heyrovsky and P. Zuman, ‘Practical Polarography’ (Academic Press, New York, 1950).
R.N. Jones and C. Sandorfy, The Application of Molecular Structure in ‘Technique of Organic Chemistry’ Vol. IX, edited by A. Weissberger (Interscience, London, 1956), p. 463.
C. Sharad Mishra and A. Ram Mishra, J. Electrochem. Soc. India 39 (1990) 51.
A. Weissenberger, (Ed.), ‘Heterocyclic Compounds with Indole and Carbazole Systems’ (Wiley Interscience, New York, 1954).
A. Renuka and K. Shakuntala, Bioelectrochem. Bioenergetics 19 (1988) 161.
R.J.P. Williams and J.J.R.F. Da Silva, in ‘New Trends in Bio-Inorganic Chemistry’ edited by R.J.P. Williams and J.J.R.F. Da Silva (Academic Press, New York, 1978) Chapter 5.
D.M. Daniel and Wayner, Bioelectrochem. Bioenergetics 18 (1987) 219.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Renuka, R. 2-Nitrophenylpyruvic acid as a cathode material in a magnesium/zinc-based primary battery. Journal of Applied Electrochemistry 30, 483–490 (2000). https://doi.org/10.1023/A:1003974916237
Issue Date:
DOI: https://doi.org/10.1023/A:1003974916237