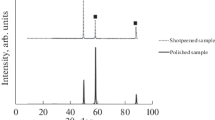
Abstract
Sodium hexanoate is proposed as corrosion inhibitor for copper when the metal is situated in an aggressive aqueous or gaseous environment. The efficiency of the inhibitor, measured by electrochemical techniques in the ASTM 1384 standard solution, depends on the hexanoate concentration. Copper foils immersed in a (0.1 M) sodium hexanoate conditioning bath, and later exposed to a strongly oxidizing gliding arc plasma in humid air, yield oxide layers, the thickness and the nature of which differ from the unconditioned samples since the voltammograms show no CuO at the surface. The organic salt thus limits the oxidation process induced by the gaseous species. The organic layer remains active for at least 30 min. For longer exposures, the salt begins to degrade to carbon dioxide, which makes the plasma treatment a useful tool to clean metallic surfaces.
Similar content being viewed by others
References
M. Poubaix, ‘Atlas of Electrochemical Equilibria in Aqueous Solutions’, (Pergamon, New York, 1966).
R.R. Thomas, V.A. Brusic and B.M. Rush, J. Electrochem. Soc. 139 (1992) 678.
C. Fiaud, Proceedings of the Int. Symposium on ‘Control of Copper and Copper Alloys Oxidation’, Rouen, France, 1992, p. 97.
F.P. Eng and H. Ishida, J. Appl. Polym. Sci. 32 (1986) 5023.
F.P. Eng and H. Ishida, J. Mater. Sci. 21 (1986) 1561.
V. Brusic, M.A. Frisch, B.N. Eldrige, F.P. Novak, F.B. Kaufman, B.M. Rush and G.S. Frankel, J. Electrochem. Soc. 138 (1991) 2253.
A. Shaban, J. Telegdi, S. Alexandre and E. Kalman, Proc. Eurocorr. 94, Bournemouth, UK, 1994, vol. 1, p. 112.
A.G. Kumbhar, S.V. Narasimhan and P.K. Mathur, Indian. J. Chem. 33A (1994) 47.
N. Bellakhal, K. Draou and J.L. Brisset, J. Appl. Electrochem. 27 (1997) 414.
N. Bellakhal, K. Draou, B.G. Chéron and J. L. Brisset, Mat. Sci. Eng B 41 (1996) 206.
K. Draou, N. Bellakhal, B.G. Chéron and J. L. Brisset, Mat. Chem. Phys . 58 (1999) 212.
H. Lesueur, A. Czernichowski and J. Chapelle, French patent 2 639 172 (1988).
A. Fridman, R. Petrousov, J. Chapelle, L.M. Carnier, A. Czerni-chowski, H. Lesueur and J. Stevefelt, J. Phys. III Fr 4 (1994) 1449.
B. Benstaali, D. Moussa, A. Addou and J.L. Brisset, Europ. Phys. J. Appl. Phys. 4 (1998) 171.
F. Moras and J.L. Brisset, Proc. 5th International Symposium on ‘High Pressure Low Temperature Plasma Chem. “Hakone V”’, Milovy, Czech Republic, 1996, p. 87.
B. Benstaali, P. Boubert, B.G. Chéron, A. Addou, J.L. Brisset, accepted to Plasma Chem. Plasma Proc.
ASTM, D 1384–87 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bellakhal, N., Draou, K., Addou, A. et al. Cleaning of copper foil coated with sodium hexanoate as corrosion inhibitor. Journal of Applied Electrochemistry 30, 595–600 (2000). https://doi.org/10.1023/A:1003964304448
Issue Date:
DOI: https://doi.org/10.1023/A:1003964304448